当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From a Molecular 2Fe-2Se Precursor to a Highly Efficient Iron Diselenide Electrocatalyst for Overall Water Splitting
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:21:25 , DOI: 10.1002/anie.201706196 Chakadola Panda 1 , Prashanth W. Menezes 1 , Carsten Walter 1 , Shenglai Yao 1 , Matthias E. Miehlich 2 , Vitaly Gutkin 3 , Karsten Meyer 2 , Matthias Driess 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:21:25 , DOI: 10.1002/anie.201706196 Chakadola Panda 1 , Prashanth W. Menezes 1 , Carsten Walter 1 , Shenglai Yao 1 , Matthias E. Miehlich 2 , Vitaly Gutkin 3 , Karsten Meyer 2 , Matthias Driess 1
Affiliation
|
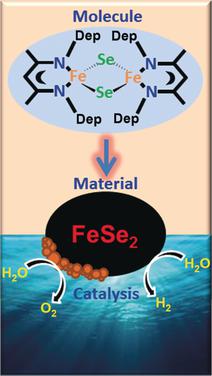
A highly active FeSe2 electrocatalyst for durable overall water splitting was prepared from a molecular 2Fe-2Se precursor. The as-synthesized FeSe2 was electrophoretically deposited on nickel foam and applied to the oxygen and hydrogen evolution reactions (OER and HER, respectively) in alkaline media. When used as an oxygen-evolution electrode, a low 245 mV overpotential was achieved at a current density of 10 mA cm−2, representing outstanding catalytic activity and stability because of Fe(OH)2/FeOOH active sites formed at the surface of FeSe2. Remarkably, the system is also favorable for the HER. Moreover, an overall water-splitting setup was fabricated using a two-electrode cell, which displayed a low cell voltage and high stability. In summary, the first iron selenide material is reported that can be used as a bifunctional electrocatalyst for the OER and HER, as well as overall water splitting.
中文翻译:

从分子2Fe-2Se前驱体到高效的二硒化铁电催化剂,用于总水分解
由分子2Fe-2Se前驱体制备了用于持久性总水分解的高活性FeSe 2电催化剂。合成后的FeSe 2电泳沉积在泡沫镍上,并在碱性介质中应用于氧和氢的放出反应(分别为OER和HER)。当用作放氧电极时,在10 mA cm -2的电流密度下可实现低245 mV的过电势,由于在FeSe表面形成了Fe(OH)2 / FeOOH活性位,因此具有出色的催化活性和稳定性。2个。值得注意的是,该系统对HER也很有利。而且,使用两电极电池制造了整体的水分解装置,其显示出低电池电压和高稳定性。总之,据报道,第一种硒化铁材料可用作OER和HER的双功能电催化剂,以及总的水分解剂。
更新日期:2017-07-20
中文翻译:

从分子2Fe-2Se前驱体到高效的二硒化铁电催化剂,用于总水分解
由分子2Fe-2Se前驱体制备了用于持久性总水分解的高活性FeSe 2电催化剂。合成后的FeSe 2电泳沉积在泡沫镍上,并在碱性介质中应用于氧和氢的放出反应(分别为OER和HER)。当用作放氧电极时,在10 mA cm -2的电流密度下可实现低245 mV的过电势,由于在FeSe表面形成了Fe(OH)2 / FeOOH活性位,因此具有出色的催化活性和稳定性。2个。值得注意的是,该系统对HER也很有利。而且,使用两电极电池制造了整体的水分解装置,其显示出低电池电压和高稳定性。总之,据报道,第一种硒化铁材料可用作OER和HER的双功能电催化剂,以及总的水分解剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号