当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Regioselectivity in the Enantioselective N-Alkylation of Indole Analogues Catalyzed by Dinuclear Zinc-ProPhenol
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:20:57 , DOI: 10.1002/anie.201705315 Barry M. Trost 1 , Elumalai Gnanamani 1 , Chao-I Joey Hung 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:20:57 , DOI: 10.1002/anie.201705315 Barry M. Trost 1 , Elumalai Gnanamani 1 , Chao-I Joey Hung 1
Affiliation
|
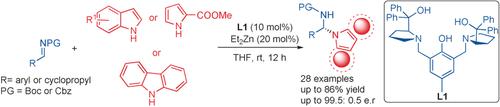
The enantioselective N-alkylation of indole and its derivatives with aldimines is efficiently catalyzed by a zinc-ProPhenol dinuclear complex under mild conditions to afford N-alkylated indole derivatives in good yield (up to 86 %) and excellent enantiomeric ratio (up to 99.5:0.5 e.r.). This method tolerates a wide array of indoles, as well as pyrrole and carbazole, to afford the corresponding N-alkylation products. The reaction can be run on a gram scale with reduced catalyst loading without impacting the efficiency. The chiral aminals were further elaborated into various chiral polyheterocyclic derivatives. The surprising stability of the chiral N-alkylation products will open new windows for asymmetric catalysis and medicinal chemistry.
中文翻译:

双核锌-苯酚催化吲哚类似物的对映选择性N-烷基化中的区域选择性控制
锌-苯酚双核络合物在温和条件下可有效催化吲哚及其衍生物与醛亚胺的对映选择性N-烷基化,从而以良好的收率(高达86%)和出色的对映体比率(高达99.5: 0.5 er)。该方法耐受各种各样的吲哚以及吡咯和咔唑,以提供相应的N-烷基化产物。该反应可以以克规模进行,催化剂负载减少,而不会影响效率。将手性缩醛进一步加工成各种手性多杂环衍生物。手性N-烷基化产物的惊人的稳定性将为不对称催化和药物化学打开新的窗口。
更新日期:2017-07-20
中文翻译:

双核锌-苯酚催化吲哚类似物的对映选择性N-烷基化中的区域选择性控制
锌-苯酚双核络合物在温和条件下可有效催化吲哚及其衍生物与醛亚胺的对映选择性N-烷基化,从而以良好的收率(高达86%)和出色的对映体比率(高达99.5: 0.5 er)。该方法耐受各种各样的吲哚以及吡咯和咔唑,以提供相应的N-烷基化产物。该反应可以以克规模进行,催化剂负载减少,而不会影响效率。将手性缩醛进一步加工成各种手性多杂环衍生物。手性N-烷基化产物的惊人的稳定性将为不对称催化和药物化学打开新的窗口。









































 京公网安备 11010802027423号
京公网安备 11010802027423号