当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bromination of Cycloparaphenylenes: Strain-Induced Site-Selective Bis-Addition and its Application for Late-Stage Functionalization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:15:48 , DOI: 10.1002/anie.201704982 Eiichi Kayahara 1 , Rui Qu 1 , Shigeru Yamago 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-19 06:15:48 , DOI: 10.1002/anie.201704982 Eiichi Kayahara 1 , Rui Qu 1 , Shigeru Yamago 1
Affiliation
|
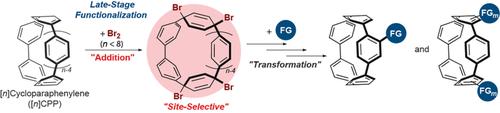
Bromination of [n]cycloparaphenylenes (CPPs) is herein reported. Small [n]CPPs (n<8) underwent a bis-bromine addition reaction with high site selectively to produce tetrabromo adducts in moderate to excellent yields. Theoretical calculations revealed that thermodynamic stability dictates both the reactivity and site selectivity of the reaction. The addition product was further converted into the octabromo product by a FeBr3-catalyzed site-selective bromination reaction. The tetra- and octabromine adducts were then transformed into mono- to tetrabromo CPPs, which were further converted into several CPP derivatives. Therefore, bromination and subsequent transformations provide a path for late-stage functionalization of CPPs.
中文翻译:

环对亚苯基的溴化:应变诱导的位点选择性双加成法及其在后期功能化中的应用
本文报道了[ n ]环对亚苯基(CPP)的溴化。较小的[ n ] CPPs(n <8)进行高位点的双溴加成反应,以中等至极好的收率选择性地生产四溴加合物。理论计算表明,热力学稳定性决定了反应的反应性和位点选择性。通过FeBr 3催化的位点选择性溴化反应将加成产物进一步转化为八溴代产物。然后将四溴和八溴胺加合物转化为单溴到四溴CPP,然后将其进一步转化为几种CPP衍生物。因此,溴化和随后的转化为CPP的后期功能化提供了一条途径。
更新日期:2017-07-20
中文翻译:

环对亚苯基的溴化:应变诱导的位点选择性双加成法及其在后期功能化中的应用
本文报道了[ n ]环对亚苯基(CPP)的溴化。较小的[ n ] CPPs(n <8)进行高位点的双溴加成反应,以中等至极好的收率选择性地生产四溴加合物。理论计算表明,热力学稳定性决定了反应的反应性和位点选择性。通过FeBr 3催化的位点选择性溴化反应将加成产物进一步转化为八溴代产物。然后将四溴和八溴胺加合物转化为单溴到四溴CPP,然后将其进一步转化为几种CPP衍生物。因此,溴化和随后的转化为CPP的后期功能化提供了一条途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号