当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Optimization of Benzopiperazines as Potent Inhibitors of BET Bromodomains
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00191 David S. Millan 1 , Katherine J. Kayser-Bricker 2 , Matthew W. Martin 1 , Adam C. Talbot 2 , Shawn E. R. Schiller 1 , Torsten Herbertz 1 , Grace L. Williams 1 , George P. Luke 2 , Stephen Hubbs 2 , Monica A. Alvarez Morales 1 , Daniel Cardillo 1 , Paul Troccolo 1 , Rachel L. Mendes 1 , Crystal McKinnon 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-07-19 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00191 David S. Millan 1 , Katherine J. Kayser-Bricker 2 , Matthew W. Martin 1 , Adam C. Talbot 2 , Shawn E. R. Schiller 1 , Torsten Herbertz 1 , Grace L. Williams 1 , George P. Luke 2 , Stephen Hubbs 2 , Monica A. Alvarez Morales 1 , Daniel Cardillo 1 , Paul Troccolo 1 , Rachel L. Mendes 1 , Crystal McKinnon 1
Affiliation
|
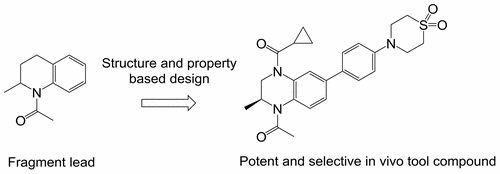
A protein structure-guided drug design approach was employed to develop small molecule inhibitors of the BET family of bromodomains that were distinct from the known (+)-JQ1 scaffold class. These efforts led to the identification of a series of substituted benzopiperazines with structural features that enable interactions with many of the affinity-driving regions of the bromodomain binding site. Lipophilic efficiency was a guiding principle in improving binding affinity alongside drug-like physicochemical properties that are commensurate with oral bioavailability. Derived from this series was tool compound FT001, which displayed potent biochemical and cellular activity, translating to excellent in vivo activity in a mouse xenograft model (MV-4-11).
中文翻译:

苯并哌嗪作为BET溴结构域有效抑制剂的设计和优化
采用蛋白质结构指导的药物设计方法来开发与已知(+)-JQ1支架类不同的bromodomains BET家族的小分子抑制剂。这些努力导致鉴定了一系列具有结构特征的取代的苯并哌嗪,所述结构特征使得能够与溴结构域结合位点的许多亲和力驱动区域相互作用。亲脂性效率是改善结合亲和力以及与口服生物利用度相当的类似药物的理化性质的指导原则。从该系列中衍生出工具化合物FT001,该化合物在小鼠异种移植模型(MV-4-11)中表现出强大的生化和细胞活性,可转化为出色的体内活性。
更新日期:2017-07-20
中文翻译:

苯并哌嗪作为BET溴结构域有效抑制剂的设计和优化
采用蛋白质结构指导的药物设计方法来开发与已知(+)-JQ1支架类不同的bromodomains BET家族的小分子抑制剂。这些努力导致鉴定了一系列具有结构特征的取代的苯并哌嗪,所述结构特征使得能够与溴结构域结合位点的许多亲和力驱动区域相互作用。亲脂性效率是改善结合亲和力以及与口服生物利用度相当的类似药物的理化性质的指导原则。从该系列中衍生出工具化合物FT001,该化合物在小鼠异种移植模型(MV-4-11)中表现出强大的生化和细胞活性,可转化为出色的体内活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号