当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kumada–Corriu Heteroaryl Cross-Coupling for Synthesis of a Pharmaceutical Intermediate: Comparison of Batch Versus Continuous Reaction Modes
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1021/acs.oprd.7b00185 Xin Linghu 1 , Nicholas Wong 1 , Vera Jost 2 , Serena Fantasia 2 , C. Gregory Sowell 1 , Francis Gosselin 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-07-18 00:00:00 , DOI: 10.1021/acs.oprd.7b00185 Xin Linghu 1 , Nicholas Wong 1 , Vera Jost 2 , Serena Fantasia 2 , C. Gregory Sowell 1 , Francis Gosselin 1
Affiliation
|
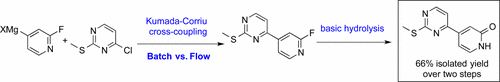
Pyridone 1, a key intermediate in the preparation of ERK inhibitor GDC-0994, was synthesized via a cross-coupling/hydrolysis sequence from commercially available starting materials. C–C bond formation was achieved via an efficient palladium catalyzed Kumada–Corriu cross-coupling reaction. However, the 4-pyridylmagnesium halide reagent generated in situ was found to be unstable at the reaction temperature, leading to inconsistent results on scale. In order to address process robustness issues associated with the cross-coupling reaction, we investigated both flow chemistry and a low temperature Kumada–Corriu coupling reaction. Finally, a basic hydrolysis process of 2-fluoropyridine was developed to avoid formation of toxic and corrosive hydrofluoric acid, resulting in a safe and scalable process toward 1.
中文翻译:

用于制药中间体合成的Kumada–Corriu杂芳基交叉偶联:批次与连续反应模式的比较
吡啶酮1是制备ERK抑制剂GDC-0994的关键中间体,它是通过交叉偶联/水解序列由可商购的起始原料合成的。通过有效的钯催化的Kumada-Corriu交叉偶联反应可实现C-C键的形成。但是,4-吡啶基卤化镁试剂是原位生成的发现在反应温度下不稳定,导致规模结果不一致。为了解决与交叉偶联反应相关的过程稳健性问题,我们研究了流化学和低温Kumada-Corriu偶联反应。最后,开发了2-氟吡啶的基本水解过程,以避免形成有毒和腐蚀性的氢氟酸,从而实现了向1的安全且可扩展的过程。
更新日期:2017-07-19
中文翻译:

用于制药中间体合成的Kumada–Corriu杂芳基交叉偶联:批次与连续反应模式的比较
吡啶酮1是制备ERK抑制剂GDC-0994的关键中间体,它是通过交叉偶联/水解序列由可商购的起始原料合成的。通过有效的钯催化的Kumada-Corriu交叉偶联反应可实现C-C键的形成。但是,4-吡啶基卤化镁试剂是原位生成的发现在反应温度下不稳定,导致规模结果不一致。为了解决与交叉偶联反应相关的过程稳健性问题,我们研究了流化学和低温Kumada-Corriu偶联反应。最后,开发了2-氟吡啶的基本水解过程,以避免形成有毒和腐蚀性的氢氟酸,从而实现了向1的安全且可扩展的过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号