当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TMSCF3 as a Convenient Source of CF2=CF2 for Pentafluoroethylation, (Aryloxy)tetrafluoroethylation, and Tetrafluoroethylation
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-18 02:27:47 , DOI: 10.1002/anie.201705734 Lingchun Li 1 , Chuanfa Ni 1 , Qiqiang Xie 1 , Mingyou Hu 1 , Fei Wang 1 , Jinbo Hu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-18 02:27:47 , DOI: 10.1002/anie.201705734 Lingchun Li 1 , Chuanfa Ni 1 , Qiqiang Xie 1 , Mingyou Hu 1 , Fei Wang 1 , Jinbo Hu 1
Affiliation
|
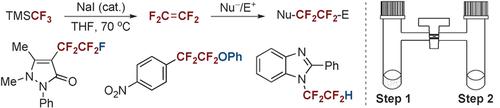
A new method for the on-site preparation of tetrafluoroethylene (TFE) and a procedure for its efficient use in pentafluoroethylation by fluoride addition were developed by using a simple two-chamber system. The on-site preparation of TFE was accomplished by dimerization of difluorocarbene derived from (trifluoromethyl)trimethylsilane (TMSCF3) under mild conditions. Other fluoroalkylation reactions, such as (aryloxy)tetrafluoroethylation and tetrafluoroethylation processes, were also achieved using a similar approach. This work not only demonstrates a convenient and safe approach for the generation and use of TFE in academic laboratories, but also provides a new strategy for pentafluoroethylation.
中文翻译:

TMSCF3作为五氟乙基化,(芳氧基)四氟乙基化和四氟乙基化的CF2 = CF2的便捷来源
通过使用简单的两腔系统,开发了一种现场制备四氟乙烯(TFE)的新方法及其通过添加氟化物有效地用于五氟乙基化的方法。TFE的现场制备是通过在温和条件下将衍生自(三氟甲基)三甲基硅烷(TMSCF 3)的二氟卡宾进行二聚完成的。其他的氟烷基化反应,例如(芳氧基)四氟乙基化和四氟乙基化过程,也可以使用类似的方法来实现。这项工作不仅证明了在实验室中生成和使用TFE的便捷安全方法,而且为五氟乙基化提供了新的策略。
更新日期:2017-07-19
中文翻译:

TMSCF3作为五氟乙基化,(芳氧基)四氟乙基化和四氟乙基化的CF2 = CF2的便捷来源
通过使用简单的两腔系统,开发了一种现场制备四氟乙烯(TFE)的新方法及其通过添加氟化物有效地用于五氟乙基化的方法。TFE的现场制备是通过在温和条件下将衍生自(三氟甲基)三甲基硅烷(TMSCF 3)的二氟卡宾进行二聚完成的。其他的氟烷基化反应,例如(芳氧基)四氟乙基化和四氟乙基化过程,也可以使用类似的方法来实现。这项工作不仅证明了在实验室中生成和使用TFE的便捷安全方法,而且为五氟乙基化提供了新的策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号