当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Field NMR Spectroscopy Reveals Aromaticity-Modulated Hydrogen Bonding in Heterocycles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-18 02:25:33 , DOI: 10.1002/anie.201705023 Tayeb Kakeshpour 1 , John P. Bailey 2 , Madison R. Jenner 1 , Darya E. Howell 1 , Richard J. Staples 1 , Daniel Holmes 1 , Judy I. Wu 3 , James E. Jackson 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-18 02:25:33 , DOI: 10.1002/anie.201705023 Tayeb Kakeshpour 1 , John P. Bailey 2 , Madison R. Jenner 1 , Darya E. Howell 1 , Richard J. Staples 1 , Daniel Holmes 1 , Judy I. Wu 3 , James E. Jackson 1
Affiliation
|
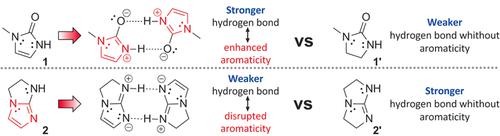
From DNA base pairs to drug–receptor binding, hydrogen (H-)bonding and aromaticity are common features of heterocycles. Herein, the interplay of these bonding aspects is explored. H-bond strength modulation due to enhancement or disruption of aromaticity of heterocycles is experimentally revealed by comparing homodimer H-bond energies of aromatic heterocycles with analogs that have the same H-bonding moieties but lack cyclic π-conjugation. NMR studies of dimerization in C6D6 find aromaticity-modulated H-bonding (AMHB) energy effects of approximately ±30 %, depending on whether they enhance or weaken aromatic delocalization. The attendant ring current perturbations expected from such modulation are confirmed by chemical shift changes in both observed ring C−H and calculated nucleus-independent sites. In silico modeling confirms that AMHB effects outweigh those of hybridization or dipole–dipole interaction.
中文翻译:

高场核磁共振波谱揭示了杂环中的芳香族调节氢键。
从DNA碱基对到药物-受体结合,氢(H-)键和芳香性是杂环的常见特征。在本文中,探索了这些结合方面的相互作用。通过比较芳香族杂环的同二聚体H键能与具有相同H键部分但缺乏环状π共轭的类似物,通过实验揭示了由于杂环的芳香性增强或破坏引起的H键强度调节。在C 6 D 6中的二聚化的NMR研究发现芳烃调节的H键(AMHB)能量效应约为±30%,具体取决于它们是增强还是减弱了芳烃的离域作用。通过观察到的环CH和计算出的与核无关的位点中的化学位移变化,可以确认这种调制带来的伴随的环电流扰动。计算机模拟表明,AMHB的影响大于杂交或偶极-偶极相互作用的影响。
更新日期:2017-07-19
中文翻译:

高场核磁共振波谱揭示了杂环中的芳香族调节氢键。
从DNA碱基对到药物-受体结合,氢(H-)键和芳香性是杂环的常见特征。在本文中,探索了这些结合方面的相互作用。通过比较芳香族杂环的同二聚体H键能与具有相同H键部分但缺乏环状π共轭的类似物,通过实验揭示了由于杂环的芳香性增强或破坏引起的H键强度调节。在C 6 D 6中的二聚化的NMR研究发现芳烃调节的H键(AMHB)能量效应约为±30%,具体取决于它们是增强还是减弱了芳烃的离域作用。通过观察到的环CH和计算出的与核无关的位点中的化学位移变化,可以确认这种调制带来的伴随的环电流扰动。计算机模拟表明,AMHB的影响大于杂交或偶极-偶极相互作用的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号