当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Convergent Synthesis of Functionalized Alkenyl Halides through Cobalt(III)-Catalyzed Three-Component C−H Bond Addition
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 08:18:37 , DOI: 10.1002/anie.201705817 Jeffrey A. Boerth 1 , Jonathan A. Ellman 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 08:18:37 , DOI: 10.1002/anie.201705817 Jeffrey A. Boerth 1 , Jonathan A. Ellman 1
Affiliation
|
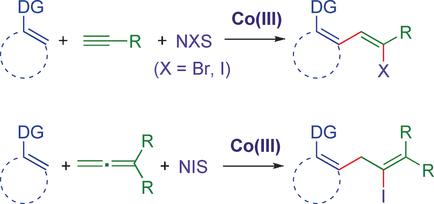
A CoIII-catalyzed three-component coupling of C(sp2)−H bonds, alkynes, and halogenating agents to give alkenyl halides is reported. This transformation proceeds with high regio- and diastereoselectivity, and is effective for a broad range of aryl and alkyl terminal alkynes. Diverse C−H bond partners also exhibit good reactivity for a range of heteroaryl and aryl systems as well as synthetically useful secondary and tertiary amide, urea, and pyrazole directing groups. This multicomponent transformation is also compatible with allenes in place of alkynes to furnish tetrasubstituted alkenyl halides, showcasing the first halo-arylation of allenes.
中文翻译:

通过钴(III)催化的三组分CH键加合聚合合成官能化的烯基卤化物
据报道,Co III催化了C(sp 2)-H键,炔烃和卤化剂的三组分偶联,从而得到烯基卤化物。该转化以高区域选择性和非对映选择性进行,并且对于宽范围的芳基和烷基末端炔烃有效。不同的CH键对也对一系列杂芳基和芳基系统以及合成有用的仲和叔酰胺,脲和吡唑导向基团表现出良好的反应性。该多组分转化还与取代炔烃的丙二烯相容,以提供四取代的烯基卤化物,显示了丙二烯的首次卤代芳基化。
更新日期:2017-07-17
中文翻译:

通过钴(III)催化的三组分CH键加合聚合合成官能化的烯基卤化物
据报道,Co III催化了C(sp 2)-H键,炔烃和卤化剂的三组分偶联,从而得到烯基卤化物。该转化以高区域选择性和非对映选择性进行,并且对于宽范围的芳基和烷基末端炔烃有效。不同的CH键对也对一系列杂芳基和芳基系统以及合成有用的仲和叔酰胺,脲和吡唑导向基团表现出良好的反应性。该多组分转化还与取代炔烃的丙二烯相容,以提供四取代的烯基卤化物,显示了丙二烯的首次卤代芳基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号