当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analyzing Reaction Rates with the Distortion/Interaction-Activation Strain Model
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 08:18:03 , DOI: 10.1002/anie.201701486 F. Matthias Bickelhaupt 1, 2 , Kendall N. Houk 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 08:18:03 , DOI: 10.1002/anie.201701486 F. Matthias Bickelhaupt 1, 2 , Kendall N. Houk 3
Affiliation
|
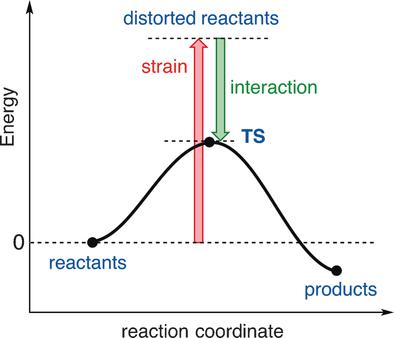
The activation strain or distortion/interaction model is a tool to analyze activation barriers that determine reaction rates. For bimolecular reactions, the activation energies are the sum of the energies to distort the reactants into geometries they have in transition states plus the interaction energies between the two distorted molecules. The energy required to distort the molecules is called the activation strain or distortion energy. This energy is the principal contributor to the activation barrier. The transition state occurs when this activation strain is overcome by the stabilizing interaction energy. Following the changes in these energies along the reaction coordinate gives insights into the factors controlling reactivity. This model has been applied to reactions of all types in both organic and inorganic chemistry, including substitutions and eliminations, cycloadditions, and several types of organometallic reactions.
中文翻译:

用变形/相互作用激活应变模型分析反应速率
激活应变或变形/相互作用模型是分析确定反应速率的激活障碍的工具。对于双分子反应,活化能是将反应物扭曲成处于过渡态的几何形状的能量之和,再加上两个扭曲的分子之间的相互作用能。使分子变形所需的能量称为活化应变或变形能。该能量是激活屏障的主要贡献者。当此激活应变被稳定的相互作用能克服时,就会出现过渡状态。跟随这些能量沿着反应坐标的变化,可以深入了解控制反应性的因素。该模型已应用于有机和无机化学中的所有类型的反应,
更新日期:2017-07-17
中文翻译:

用变形/相互作用激活应变模型分析反应速率
激活应变或变形/相互作用模型是分析确定反应速率的激活障碍的工具。对于双分子反应,活化能是将反应物扭曲成处于过渡态的几何形状的能量之和,再加上两个扭曲的分子之间的相互作用能。使分子变形所需的能量称为活化应变或变形能。该能量是激活屏障的主要贡献者。当此激活应变被稳定的相互作用能克服时,就会出现过渡状态。跟随这些能量沿着反应坐标的变化,可以深入了解控制反应性的因素。该模型已应用于有机和无机化学中的所有类型的反应,











































 京公网安备 11010802027423号
京公网安备 11010802027423号