当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Self‐Sacrificing N‐Methyltransferase Is the Precursor of the Fungal Natural Product Omphalotin
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 , DOI: 10.1002/anie.201703488 Sascha Ramm 1 , Bartlomiej Krawczyk 1 , Agnes Mühlenweg 1 , Annette Poch 1 , Eva Mösker 1 , Roderich D. Süssmuth 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-07-17 , DOI: 10.1002/anie.201703488 Sascha Ramm 1 , Bartlomiej Krawczyk 1 , Agnes Mühlenweg 1 , Annette Poch 1 , Eva Mösker 1 , Roderich D. Süssmuth 1
Affiliation
|
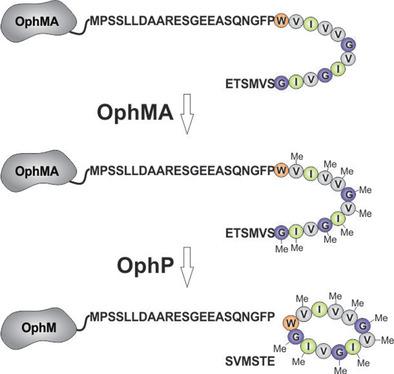
Research on ribosomally synthesized and posttranslationally modified peptides (RiPPs) has led to an increasing understanding of biosynthetic mechanisms, mostly drawn from bacterial examples. In contrast, reports on RiPPs from fungal producers, apart from the amanitins and phalloidins, are still scarce. The fungal cyclopeptide omphalotin A carries multiple N‐methylations on the peptide backbone, a modification previously known only from nonribosomal peptides. Mining the genome of the omphalotin‐producing fungus for a precursor peptide led to the identification of two biosynthesis genes, one encoding a methyltransferase OphMA that catalyzes the automethylation of its C‐terminus, which is then released and cyclized by the protease OphP. Our findings suggest a novel biosynthesis mechanism for a RiPP in which a modifying enzyme bears its own precursor peptide.
中文翻译:

一种自我牺牲的N-甲基转移酶是真菌天然产物Ophphalotin的前身
对核糖体合成的和翻译后修饰的肽(RiPPs)的研究已导致人们对生物合成机制的了解不断增加,这些生物合成机制大多来自细菌实例。相比之下,除甘露素和鬼笔环肽外,真菌生产者对RiPPs的报道仍然很少。真菌环肽omphalotin A在肽主链上带有多个N-甲基化,以前只有非核糖体肽才知道这种修饰。开采产生前体肽的产卵菌素的真菌基因组导致鉴定出两个生物合成基因,一个编码甲基转移酶OphMA,催化其C末端的自甲基化,然后被蛋白酶OphP释放并环化。
更新日期:2017-07-17
中文翻译:

一种自我牺牲的N-甲基转移酶是真菌天然产物Ophphalotin的前身
对核糖体合成的和翻译后修饰的肽(RiPPs)的研究已导致人们对生物合成机制的了解不断增加,这些生物合成机制大多来自细菌实例。相比之下,除甘露素和鬼笔环肽外,真菌生产者对RiPPs的报道仍然很少。真菌环肽omphalotin A在肽主链上带有多个N-甲基化,以前只有非核糖体肽才知道这种修饰。开采产生前体肽的产卵菌素的真菌基因组导致鉴定出两个生物合成基因,一个编码甲基转移酶OphMA,催化其C末端的自甲基化,然后被蛋白酶OphP释放并环化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号