当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polypeptide-Grafted Nanodiamonds for Controlled Release of Melittin to Treat Breast Cancer
ACS Macro Letters ( IF 5.1 ) Pub Date : 2017-07-14 00:00:00 , DOI: 10.1021/acsmacrolett.7b00389 Haiwang Lai 1 , Fan Chen 1 , Mingxia Lu 1 , Martina H. Stenzel 1 , Pu Xiao 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2017-07-14 00:00:00 , DOI: 10.1021/acsmacrolett.7b00389 Haiwang Lai 1 , Fan Chen 1 , Mingxia Lu 1 , Martina H. Stenzel 1 , Pu Xiao 1
Affiliation
|
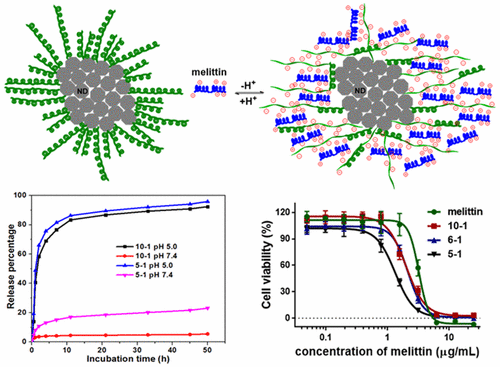
A peptide vector consisting of nanodiamonds (NDs) and PEGylated polyglutamic acid ([email protected]co-PLGA) has been designed and developed. The negative charges at the surface of the vector were exploited to bind a positively charged peptide drug melittin via electrostatic interaction. The surface was saturated when the weight ratio of [email protected]co-PLGA to melittin (MEL) was 5 to 1. The desorption of melittin from the surface was controlled by pH, with almost no melittin released from the nanoparticles under physiological pH conditions in 2 days. However, steady release was detected in an acidic environment. The preserved structure and activity of bound melittin were demonstrated by the HPLC and 2D MCF-7 cell culture models, respectively. The bound melittin exhibited improved toxicity toward MCF-7 cells dependent on the concentration of MEL in NDs. Our results suggested that the negatively charged polymer-coated NDs were able to release the cargo upon exposure to breast cancer cells.
中文翻译:

多肽接枝的纳米金刚石可控制释放的蜂毒蛋白治疗乳腺癌。
已经设计和开发了由纳米金刚石(NDs)和聚乙二醇化聚谷氨酸([电子邮件保护] co -PLGA)组成的肽载体。利用载体表面的负电荷通过静电相互作用结合带正电荷的肽药物蜂毒肽。当[email protected] co的重量比达到一定值时,表面饱和-PLGA与蜂毒素(MEL)的比为5:1。通过pH控制蜂毒素从表面的解吸,在2天的生理pH条件下,几乎没有蜂毒素从纳米颗粒中释放出来。但是,在酸性环境中检测到稳定释放。结合的蜂毒蛋白的保留结构和活性分别通过HPLC和2D MCF-7细胞培养模型证明。结合的蜂毒蛋白对MCF-7细胞表现出改善的毒性,这取决于NDs中MEL的浓度。我们的结果表明,带负电荷的聚合物涂层ND能够在暴露于乳腺癌细胞后释放出货物。
更新日期:2017-07-14
中文翻译:

多肽接枝的纳米金刚石可控制释放的蜂毒蛋白治疗乳腺癌。
已经设计和开发了由纳米金刚石(NDs)和聚乙二醇化聚谷氨酸([电子邮件保护] co -PLGA)组成的肽载体。利用载体表面的负电荷通过静电相互作用结合带正电荷的肽药物蜂毒肽。当[email protected] co的重量比达到一定值时,表面饱和-PLGA与蜂毒素(MEL)的比为5:1。通过pH控制蜂毒素从表面的解吸,在2天的生理pH条件下,几乎没有蜂毒素从纳米颗粒中释放出来。但是,在酸性环境中检测到稳定释放。结合的蜂毒蛋白的保留结构和活性分别通过HPLC和2D MCF-7细胞培养模型证明。结合的蜂毒蛋白对MCF-7细胞表现出改善的毒性,这取决于NDs中MEL的浓度。我们的结果表明,带负电荷的聚合物涂层ND能够在暴露于乳腺癌细胞后释放出货物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号