Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Real-Time Multiplex Kinase Phosphorylation Sensors in Living Cells
ACS Sensors ( IF 8.9 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1021/acssensors.7b00359 Nur P. Damayanti 1 , Kevin Buno , Yi Cui , Sherry L. Voytik-Harbin , Roberto Pili 1 , Jennifer Freeman 2 , Joseph M. K. Irudayaraj
ACS Sensors ( IF 8.9 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1021/acssensors.7b00359 Nur P. Damayanti 1 , Kevin Buno , Yi Cui , Sherry L. Voytik-Harbin , Roberto Pili 1 , Jennifer Freeman 2 , Joseph M. K. Irudayaraj
Affiliation
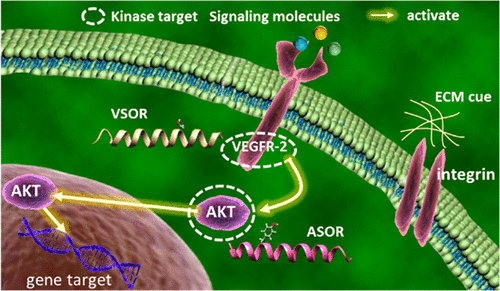
|
Phosphorylation is an important post-translational modification implicated in cellular signaling and regulation. However, current methods to study protein phosphorylation by various kinases lack spatiotemporal resolution or the ability to simultaneously observe in real time the activity of multiple kinases in live cells. We present a peptide biosensor strategy with time correlated single photon counting-fluorescence lifetime imaging (TCSPC-FLIM) to interrogate the spatial and temporal dynamics of VEGFR-2 and AKT phosphorylation activity in real time in live 2D and 3D cell culture models at single cell resolution. By recording the increase in fluorescence lifetime due to a change in the solvatochromic environment of the sensor upon phosphorylation, we demonstrate that spatiotemporal maps of protein kinase activity can be obtained. Our results suggest that fluorescence lifetime imaging of peptide biosensors can be effectively and specifically used to monitor and quantify phosphorylation of multiple kinases in live cells.
中文翻译:

活细胞中的实时多重激酶磷酸化传感器
磷酸化是重要的翻译后修饰,与细胞信号传导和调控有关。然而,目前研究各种激酶的蛋白磷酸化的方法缺乏时空分辨率或同时实时观察活细胞中多种激酶活性的能力。我们提出了一种与时间相关的单光子计数-荧光寿命成像(TCSPC-FLIM)的肽生物传感器策略,以实时在单个细胞的实时2D和3D细胞培养模型中实时询问VEGFR-2和AKT磷酸化活性的时空动态解析度。通过记录由于磷酸化后传感器的溶剂变色环境的变化而导致的荧光寿命的增加,我们证明了可以获得蛋白激酶活性的时空图。
更新日期:2017-07-14
中文翻译:

活细胞中的实时多重激酶磷酸化传感器
磷酸化是重要的翻译后修饰,与细胞信号传导和调控有关。然而,目前研究各种激酶的蛋白磷酸化的方法缺乏时空分辨率或同时实时观察活细胞中多种激酶活性的能力。我们提出了一种与时间相关的单光子计数-荧光寿命成像(TCSPC-FLIM)的肽生物传感器策略,以实时在单个细胞的实时2D和3D细胞培养模型中实时询问VEGFR-2和AKT磷酸化活性的时空动态解析度。通过记录由于磷酸化后传感器的溶剂变色环境的变化而导致的荧光寿命的增加,我们证明了可以获得蛋白激酶活性的时空图。



























 京公网安备 11010802027423号
京公网安备 11010802027423号