当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
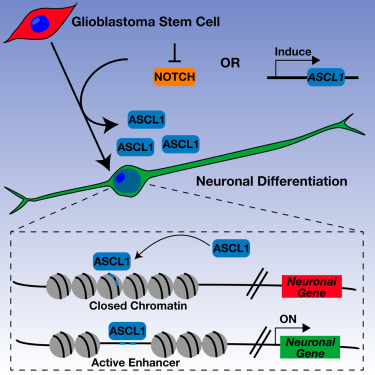
ASCL1 Reorganizes Chromatin to Direct Neuronal Fate and Suppress Tumorigenicity of Glioblastoma Stem Cells.
Cell Stem Cell ( IF 23.9 ) Pub Date : 2017-08-03 , DOI: 10.1016/j.stem.2017.06.004 Nicole I. Park , Paul Guilhamon , Kinjal Desai , Rochelle F. McAdam , Ellen Langille , Madlen O’Connor , Xiaoyang Lan , Heather Whetstone , Fiona J. Coutinho , Robert J. Vanner , Erick Ling , Panagiotis Prinos , Lilian Lee , Hayden Selvadurai , Gurnit Atwal , Michelle Kushida , Ian D. Clarke , Veronique Voisin , Michael D. Cusimano , Mark Bernstein , Sunit Das , Gary Bader , Cheryl H. Arrowsmith , Stephane Angers , Xi Huang , Mathieu Lupien , Peter B. Dirks
Cell Stem Cell ( IF 23.9 ) Pub Date : 2017-08-03 , DOI: 10.1016/j.stem.2017.06.004 Nicole I. Park , Paul Guilhamon , Kinjal Desai , Rochelle F. McAdam , Ellen Langille , Madlen O’Connor , Xiaoyang Lan , Heather Whetstone , Fiona J. Coutinho , Robert J. Vanner , Erick Ling , Panagiotis Prinos , Lilian Lee , Hayden Selvadurai , Gurnit Atwal , Michelle Kushida , Ian D. Clarke , Veronique Voisin , Michael D. Cusimano , Mark Bernstein , Sunit Das , Gary Bader , Cheryl H. Arrowsmith , Stephane Angers , Xi Huang , Mathieu Lupien , Peter B. Dirks
|
Glioblastomas exhibit a hierarchical cellular organization, suggesting that they are driven by neoplastic stem cells that retain partial yet abnormal differentiation potential. Here, we show that a large subset of patient-derived glioblastoma stem cells (GSCs) express high levels of Achaete-scute homolog 1 (ASCL1), a proneural transcription factor involved in normal neurogenesis. ASCL1hi GSCs exhibit a latent capacity for terminal neuronal differentiation in response to inhibition of Notch signaling, whereas ASCL1lo GSCs do not. Increasing ASCL1 levels in ASCL1lo GSCs restores neuronal lineage potential, promotes terminal differentiation, and attenuates tumorigenicity. ASCL1 mediates these effects by functioning as a pioneer factor at closed chromatin, opening new sites to activate a neurogenic gene expression program. Directing GSCs toward terminal differentiation may provide therapeutic applications for a subset of GBM patients and strongly supports efforts to restore differentiation potential in GBM and other cancers.
中文翻译:

ASCL1重组染色质直接神经元命运,并抑制胶质母细胞瘤干细胞的致瘤性。
胶质母细胞瘤表现出分级的细胞结构,表明它们由保留部分但异常分化潜能的赘生性干细胞驱动。在这里,我们显示,大量的患者源性胶质母细胞瘤干细胞(GSC)表达高水平的Achaete-scute同源物1(ASCL1),这是参与正常神经发生的前神经转录因子。ASCL1 hi GSC对Notch信号的抑制具有潜在的终末神经元分化能力,而ASCL1 lo GSC没有。在ASCL1 lo中增加ASCL1级别GSCs恢复神经元谱系潜力,促进终末分化,并减弱致瘤性。ASCL1通过充当封闭染色质的先驱因子,打开新位点以激活神经原性基因表达程序来介导这些作用。将GSC引导至终末分化可能为部分GBM患者提供治疗应用,并大力支持恢复GBM和其他癌症分化潜能的努力。
更新日期:2017-07-14
中文翻译:

ASCL1重组染色质直接神经元命运,并抑制胶质母细胞瘤干细胞的致瘤性。
胶质母细胞瘤表现出分级的细胞结构,表明它们由保留部分但异常分化潜能的赘生性干细胞驱动。在这里,我们显示,大量的患者源性胶质母细胞瘤干细胞(GSC)表达高水平的Achaete-scute同源物1(ASCL1),这是参与正常神经发生的前神经转录因子。ASCL1 hi GSC对Notch信号的抑制具有潜在的终末神经元分化能力,而ASCL1 lo GSC没有。在ASCL1 lo中增加ASCL1级别GSCs恢复神经元谱系潜力,促进终末分化,并减弱致瘤性。ASCL1通过充当封闭染色质的先驱因子,打开新位点以激活神经原性基因表达程序来介导这些作用。将GSC引导至终末分化可能为部分GBM患者提供治疗应用,并大力支持恢复GBM和其他癌症分化潜能的努力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号