当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Potential Role of Criegee Intermediates in Nighttime Atmospheric Chemistry. A Modeling Study
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-06-26 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00044 Daphne Meidan 1 , Steven S. Brown 2, 3 , Yinon Rudich 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2017-06-26 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00044 Daphne Meidan 1 , Steven S. Brown 2, 3 , Yinon Rudich 1
Affiliation
|
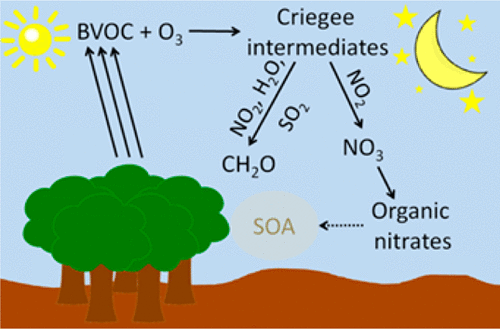
We evaluate the role of Criegee intermediates (CI) from ozonolysis of alkenes on nighttime chemistry in areas impacted by ozone and high emissions of biogenic volatile organic compounds, for example, the Southeast United States, using the Master Chemical Mechanism. Criegee reactions with NO2 may be an alternate source of NO3. Reactions of CI with NO3 have not been investigated but could influence NOx recycling. Evaluation of these reactions depends on recently measured rate constants for CI reactions with water vapor, NO2, and other trace gases. We vary the CI rate coefficients with NO2 and H2O and explore a range of initial conditions. We find that the CI production has the largest effects at low NO2 (<1 ppbv), high isoprene (10 ppbv) and low RH (<50%). At higher RH that is characteristic of the southeast U.S. and other high biogenic emitting regions, the effects are negligible using current literature values for CI rate constants with H2O. Under conditions for which CI reactions affect nighttime NO3 chemistry in the model, NO3 production from CI reaction with NO2 increases by up to 70% and 47% at 10% and 50% relative humidity (RH), respectively, but are negligible (2%) at 80% RH. Organic nitrate formation is highly correlated with NO3 production and increases up to 75%, 34%, and 1% at 10%, 50%, and 80% RH, respectively. Including CI chemistry increases formaldehyde production under all conditions. In addition to the rate constants for CI radicals with water vapor, model simulations are also sensitive to the rate constant for CI decomposition and to the yield of NO3 from CI reaction with NO2, which are uncertain.
中文翻译:

Criegee中间体在夜间大气化学中的潜在作用。建模研究
我们使用主化学机制,评估了受臭氧和高排放生物源性挥发性有机化合物(例如美国东南部)影响的地区,烯烃的臭氧分解对夜间化学反应的Criegee中间体(CI)的作用。激进分子与NO 2的反应可能是NO 3的替代来源。还没有研究CI与NO 3的反应,但可能会影响NO x的再循环。对这些反应的评估取决于最近测得的与水蒸气,NO 2和其他痕量气体进行CI反应的速率常数。我们用NO 2和H 2改变CI率系数O并探索一系列初始条件。我们发现CI生产在低NO 2(<1 ppbv),高异戊二烯(10 ppbv)和低RH(<50%)的情况下具有最大的影响。在较高的相对湿度下(美国东南部和其他高生物成因排放区的特征),使用当前文献中有关H 2 O的CI速率常数的影响可以忽略不计。在CI反应影响模型中夜间NO 3化学的条件下,NO在10%和50%的相对湿度(RH)下,与NO 2的CI反应生成的3的产量分别增加了70%和47%,但在80%RH的情况下可忽略不计(2%)。有机硝酸盐的形成与NO 3高度相关相对湿度在10%,50%和80%时分别增加至75%,34%和1%。包括CI化学在内的所有条件下,甲醛的产生都会增加。除了带有水蒸气的CI自由基的速率常数外,模型模拟还对CI分解的速率常数以及CI与NO 2的CI反应产生的NO 3的不确定性敏感。
更新日期:2017-06-26
中文翻译:

Criegee中间体在夜间大气化学中的潜在作用。建模研究
我们使用主化学机制,评估了受臭氧和高排放生物源性挥发性有机化合物(例如美国东南部)影响的地区,烯烃的臭氧分解对夜间化学反应的Criegee中间体(CI)的作用。激进分子与NO 2的反应可能是NO 3的替代来源。还没有研究CI与NO 3的反应,但可能会影响NO x的再循环。对这些反应的评估取决于最近测得的与水蒸气,NO 2和其他痕量气体进行CI反应的速率常数。我们用NO 2和H 2改变CI率系数O并探索一系列初始条件。我们发现CI生产在低NO 2(<1 ppbv),高异戊二烯(10 ppbv)和低RH(<50%)的情况下具有最大的影响。在较高的相对湿度下(美国东南部和其他高生物成因排放区的特征),使用当前文献中有关H 2 O的CI速率常数的影响可以忽略不计。在CI反应影响模型中夜间NO 3化学的条件下,NO在10%和50%的相对湿度(RH)下,与NO 2的CI反应生成的3的产量分别增加了70%和47%,但在80%RH的情况下可忽略不计(2%)。有机硝酸盐的形成与NO 3高度相关相对湿度在10%,50%和80%时分别增加至75%,34%和1%。包括CI化学在内的所有条件下,甲醛的产生都会增加。除了带有水蒸气的CI自由基的速率常数外,模型模拟还对CI分解的速率常数以及CI与NO 2的CI反应产生的NO 3的不确定性敏感。











































 京公网安备 11010802027423号
京公网安备 11010802027423号