当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Evaluation of Novel Prodrugs of Transition State Inhibitors of Norovirus 3CL Protease
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00497 Anushka C. Galasiti Kankanamalage 1 , Yunjeong Kim 2 , Athri D. Rathnayake 1 , Kevin R. Alliston 1 , Michelle M. Butler 3 , Steven C. Cardinale 3 , Terry L. Bowlin 3 , William C. Groutas 1 , Kyeong-Ok Chang 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00497 Anushka C. Galasiti Kankanamalage 1 , Yunjeong Kim 2 , Athri D. Rathnayake 1 , Kevin R. Alliston 1 , Michelle M. Butler 3 , Steven C. Cardinale 3 , Terry L. Bowlin 3 , William C. Groutas 1 , Kyeong-Ok Chang 2
Affiliation
|
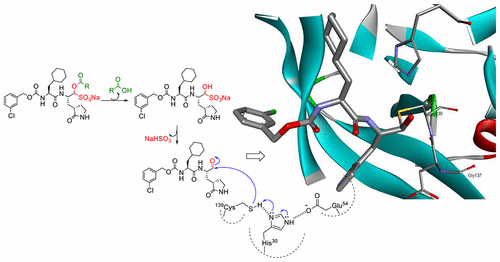
Ester and carbamate prodrugs of aldehyde bisulfite adduct inhibitors were synthesized in order to improve their pharmacokinetic and pharmacodynamic properties. The inhibitory activity of the compounds against norovirus 3C-like protease in enzyme and cell-based assays was determined. The ester and carbamate prodrugs displayed equivalent potency to those of the precursor aldehyde bisulfite adducts and precursor aldehydes. Furthermore, the rate of ester cleavage was found to be dependent on alkyl chain length. The generated prodrugs exhibited low cytotoxicity and satisfactory liver microsomes stability and plasma protein binding. The methodology described herein has wide applicability and can be extended to the bisulfite adducts of common warheads employed in the design of transition state inhibitors of serine and cysteine proteases of medical relevance.
中文翻译:

诺如病毒3CL蛋白酶的过渡态抑制剂的新型前药的设计,合成和评价
醛亚硫酸氢盐加合物抑制剂的酯和氨基甲酸酯前药被合成,以改善其药代动力学和药效学性质。在酶和基于细胞的测定中,确定了化合物对诺如病毒3C样蛋白酶的抑制活性。酯和氨基甲酸酯前药显示出与前体醛亚硫酸氢盐加合物和前体醛相同的效力。此外,发现酯的裂解速率取决于烷基链长。产生的前药显示出低细胞毒性和令人满意的肝微粒体稳定性和血浆蛋白结合。
更新日期:2017-07-11
中文翻译:

诺如病毒3CL蛋白酶的过渡态抑制剂的新型前药的设计,合成和评价
醛亚硫酸氢盐加合物抑制剂的酯和氨基甲酸酯前药被合成,以改善其药代动力学和药效学性质。在酶和基于细胞的测定中,确定了化合物对诺如病毒3C样蛋白酶的抑制活性。酯和氨基甲酸酯前药显示出与前体醛亚硫酸氢盐加合物和前体醛相同的效力。此外,发现酯的裂解速率取决于烷基链长。产生的前药显示出低细胞毒性和令人满意的肝微粒体稳定性和血浆蛋白结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号