当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer.
Cancer Cell ( IF 48.8 ) Pub Date : 2017-07-10 , DOI: 10.1016/j.ccell.2017.06.004 Surendra K Shukla 1 , Vinee Purohit 2 , Kamiya Mehla 1 , Venugopal Gunda 1 , Nina V Chaika 1 , Enza Vernucci 1 , Ryan J King 1 , Jaime Abrego 1 , Gennifer D Goode 1 , Aneesha Dasgupta 1 , Alysha L Illies 1 , Teklab Gebregiworgis 3 , Bingbing Dai 4 , Jithesh J Augustine 4 , Divya Murthy 1 , Kuldeep S Attri 1 , Oksana Mashadova 5 , Paul M Grandgenett 1 , Robert Powers 3 , Quan P Ly 6 , Audrey J Lazenby 7 , Jean L Grem 8 , Fang Yu 9 , José M Matés 10 , John M Asara 11 , Jung-Whan Kim 12 , Jordan H Hankins 13 , Colin Weekes 14 , Michael A Hollingsworth 1 , Natalie J Serkova 15 , Aaron R Sasson 16 , Jason B Fleming 4 , Jennifer M Oliveto 13 , Costas A Lyssiotis 17 , Lewis C Cantley 5 , Lyudmyla Berim 8 , Pankaj K Singh 2
Cancer Cell ( IF 48.8 ) Pub Date : 2017-07-10 , DOI: 10.1016/j.ccell.2017.06.004 Surendra K Shukla 1 , Vinee Purohit 2 , Kamiya Mehla 1 , Venugopal Gunda 1 , Nina V Chaika 1 , Enza Vernucci 1 , Ryan J King 1 , Jaime Abrego 1 , Gennifer D Goode 1 , Aneesha Dasgupta 1 , Alysha L Illies 1 , Teklab Gebregiworgis 3 , Bingbing Dai 4 , Jithesh J Augustine 4 , Divya Murthy 1 , Kuldeep S Attri 1 , Oksana Mashadova 5 , Paul M Grandgenett 1 , Robert Powers 3 , Quan P Ly 6 , Audrey J Lazenby 7 , Jean L Grem 8 , Fang Yu 9 , José M Matés 10 , John M Asara 11 , Jung-Whan Kim 12 , Jordan H Hankins 13 , Colin Weekes 14 , Michael A Hollingsworth 1 , Natalie J Serkova 15 , Aaron R Sasson 16 , Jason B Fleming 4 , Jennifer M Oliveto 13 , Costas A Lyssiotis 17 , Lewis C Cantley 5 , Lyudmyla Berim 8 , Pankaj K Singh 2
Affiliation
|
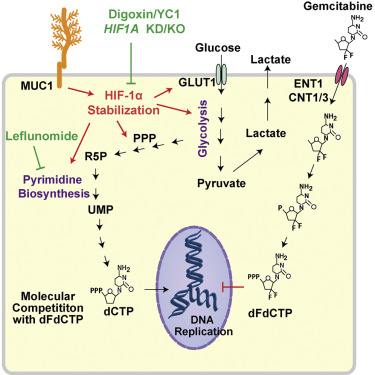
Poor response to cancer therapy due to resistance remains a clinical challenge. The present study establishes a widely prevalent mechanism of resistance to gemcitabine in pancreatic cancer, whereby increased glycolytic flux leads to glucose addiction in cancer cells and a corresponding increase in pyrimidine biosynthesis to enhance the intrinsic levels of deoxycytidine triphosphate (dCTP). Increased levels of dCTP diminish the effective levels of gemcitabine through molecular competition. We also demonstrate that MUC1-regulated stabilization of hypoxia inducible factor-1α (HIF-1α) mediates such metabolic reprogramming. Targeting HIF-1α or de novo pyrimidine biosynthesis, in combination with gemcitabine, strongly diminishes tumor burden. Finally, reduced expression of TKT and CTPS, which regulate flux into pyrimidine biosynthesis, correlates with better prognosis in pancreatic cancer patients on fluoropyrimidine analogs.
中文翻译:

MUC1 和 HIF-1alpha 信号串扰诱导合成代谢葡萄糖代谢,从而赋予吉西他滨对胰腺癌的耐药性。
由于耐药性而导致的癌症治疗反应不佳仍然是一个临床挑战。本研究建立了胰腺癌中广泛流行的吉西他滨耐药机制,即糖酵解通量增加导致癌细胞中的葡萄糖成瘾,并相应增加嘧啶生物合成,从而提高脱氧胞苷三磷酸(dCTP)的内在水平。 dCTP 水平升高会通过分子竞争降低吉西他滨的有效水平。我们还证明,MUC1 调节的缺氧诱导因子 1α (HIF-1α) 的稳定性介导了这种代谢重编程。靶向 HIF-1α 或从头嘧啶生物合成,与吉西他滨联合,可显着减轻肿瘤负荷。最后,TKT 和 CTPS 的表达减少(调节嘧啶生物合成的通量)与使用氟嘧啶类似物的胰腺癌患者更好的预后相关。
更新日期:2017-07-11
中文翻译:

MUC1 和 HIF-1alpha 信号串扰诱导合成代谢葡萄糖代谢,从而赋予吉西他滨对胰腺癌的耐药性。
由于耐药性而导致的癌症治疗反应不佳仍然是一个临床挑战。本研究建立了胰腺癌中广泛流行的吉西他滨耐药机制,即糖酵解通量增加导致癌细胞中的葡萄糖成瘾,并相应增加嘧啶生物合成,从而提高脱氧胞苷三磷酸(dCTP)的内在水平。 dCTP 水平升高会通过分子竞争降低吉西他滨的有效水平。我们还证明,MUC1 调节的缺氧诱导因子 1α (HIF-1α) 的稳定性介导了这种代谢重编程。靶向 HIF-1α 或从头嘧啶生物合成,与吉西他滨联合,可显着减轻肿瘤负荷。最后,TKT 和 CTPS 的表达减少(调节嘧啶生物合成的通量)与使用氟嘧啶类似物的胰腺癌患者更好的预后相关。









































 京公网安备 11010802027423号
京公网安备 11010802027423号