当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vivo and Mechanistic Studies on Antitumor Lead 7-Methoxy-4-(2-methylquinazolin-4-yl)-3,4-dihydroquinoxalin-2(1H)-one and Its Modification as a Novel Class of Tubulin-Binding Tumor-Vascular Disrupting Agents
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00273 Mu-Tian Cui,Li Jiang,Masuo Goto,Pei-Ling Hsu,Linna Li,Qi Zhang,Lei Wei,Shou-Jun Yuan,Ernest Hamel,Susan L. Morris-Natschke,Kuo-Hsiung Lee,Lan Xie
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-06-27 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00273 Mu-Tian Cui,Li Jiang,Masuo Goto,Pei-Ling Hsu,Linna Li,Qi Zhang,Lei Wei,Shou-Jun Yuan,Ernest Hamel,Susan L. Morris-Natschke,Kuo-Hsiung Lee,Lan Xie
|
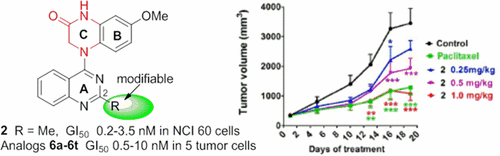
7-Methoxy-4-(2-methylquinazolin-4-yl)-3,4-dihydroquinoxalin-2(1H)-one (2), a promising anticancer lead previously identified by us, inhibited tumor growth by 62% in mice at 1.0 mg/kg without obvious signs of toxicity. Moreover, compound 2 exhibited extremely high antiproliferative activity in the NIH-NCI 60 human tumor cell line panel, with low to sub-nanomolar GI50 values (10–10 M level). It also showed a suitable balance between aqueous solubility and lipophilicity, as well as moderate metabolic stability in vivo. Mechanistic studies using Mayer’s hematoxylin and eosin and immunohistochemistry protocols on xenograft tumor tissues showed that 2 inhibited tumor cell proliferation, induced apoptosis, and disrupted tumor vasculature. Moreover, evaluation of new synthetic analogues (6a–6t) of 2 indicated that appropriate 2-substitution on the quinazoline ring could enhance antitumor activity and improve druglike properties. Compound 2 and its analogues with a 4-(2-methylquinazolin-4-yl)-3,4-dihydroquinoxalin-2(1H)-one scaffold thus represent a novel class of tubulin-binding tumor-vascular disrupting agents (tumor-VDAs) that target established blood vessels in tumors.
中文翻译:

体内和机制研究的抗肿瘤铅7-甲氧基-4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-及其修饰为一类新型的与微管蛋白结合的肿瘤-血管破坏剂
7-甲氧基-4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-一(2)是我们先前确定的有希望的抗癌药物,可抑制小鼠的肿瘤生长62%剂量为1.0 mg / kg,无明显毒性迹象。此外,化合物2在NIH-NCI 60人类肿瘤细胞系中显示出极高的抗增殖活性,其GI 50值低至亚纳摩尔级(10 – 10 M水平)。它还显示出水溶性和亲脂性之间的适当平衡,以及体内适度的代谢稳定性。使用Mayer's苏木精和曙红以及异种移植肿瘤组织的免疫组织化学方法进行的机理研究表明,2抑制肿瘤细胞增殖,诱导凋亡,破坏肿瘤血管。此外,新的合成类似物的评价(图6a - 6吨的)2所表示的喹唑啉环上适当的2-取代可提高抗肿瘤活性和改善药物样性质。因此,化合物2及其具有4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-一个支架的类似物代表了一类新型的微管蛋白结合肿瘤血管破坏剂(肿瘤-靶向肿瘤中已建立血管的VDA)。
更新日期:2017-06-27
中文翻译:

体内和机制研究的抗肿瘤铅7-甲氧基-4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-及其修饰为一类新型的与微管蛋白结合的肿瘤-血管破坏剂
7-甲氧基-4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-一(2)是我们先前确定的有希望的抗癌药物,可抑制小鼠的肿瘤生长62%剂量为1.0 mg / kg,无明显毒性迹象。此外,化合物2在NIH-NCI 60人类肿瘤细胞系中显示出极高的抗增殖活性,其GI 50值低至亚纳摩尔级(10 – 10 M水平)。它还显示出水溶性和亲脂性之间的适当平衡,以及体内适度的代谢稳定性。使用Mayer's苏木精和曙红以及异种移植肿瘤组织的免疫组织化学方法进行的机理研究表明,2抑制肿瘤细胞增殖,诱导凋亡,破坏肿瘤血管。此外,新的合成类似物的评价(图6a - 6吨的)2所表示的喹唑啉环上适当的2-取代可提高抗肿瘤活性和改善药物样性质。因此,化合物2及其具有4-(2-甲基喹唑啉-4-基)-3,4-二氢喹喔啉-2(1 H)-一个支架的类似物代表了一类新型的微管蛋白结合肿瘤血管破坏剂(肿瘤-靶向肿瘤中已建立血管的VDA)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号