当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine(III) Reagents in Radical Chemistry
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-06-21 00:00:00 , DOI: 10.1021/acs.accounts.7b00148 Xi Wang 1 , Armido Studer 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-06-21 00:00:00 , DOI: 10.1021/acs.accounts.7b00148 Xi Wang 1 , Armido Studer 1
Affiliation
|
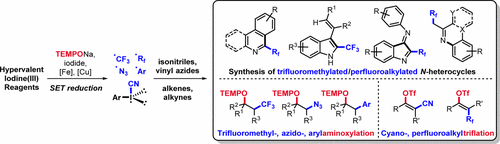
The chemistry of hypervalent iodine(III) compounds has gained great interest over the past 30 years. Hypervalent iodine(III) compounds show valuable ionic reactivity due to their high electrophilicity but also express radical reactivity as single electron oxidants for carbon and heteroatom radical generation. Looking at ionic chemistry, these iodine(III) reagents can act as electrophiles to efficiently construct C–CF3, X–CF3 (X = heteroatom), C–Rf (Rf = perfluoroalkyl), X–Rf, C–N3, C–CN, S–CN, and C–X bonds. In some cases, a Lewis or a Bronsted acid is necessary to increase their electrophilicity. In these transformations, the iodine(III) compounds react as formal “CF3+”, “Rf+”, “N3+”, “Ar+”, “CN+”, and “X+” equivalents. On the other hand, one electron reduction of the I(III) reagents opens the door to the radical world, which is the topic of this Account that focuses on radical reactivity of hypervalent iodine(III) compounds such as the Togni reagent, Zhdankin reagent, diaryliodonium salts, aryliodonium ylides, aryl(cyano)iodonium triflates, and aryl(perfluoroalkyl)iodonium triflates. Radical generation starting with I(III) reagents can also occur via thermal or light mediated homolysis of the weak hypervalent bond in such reagents. This reactivity can be used for alkane C–H functionalization. We will address important pioneering work in the area but will mainly focus on studies that have been conducted by our group over the last 5 years.
中文翻译:

自由基化学中的碘(III)试剂
在过去的30年中,高价碘(III)化合物的化学引起了人们极大的兴趣。高价碘(III)化合物由于其高亲电性而显示出宝贵的离子反应性,但作为碳和杂原子自由基生成的单电子氧化剂,也表现出自由基反应性。从离子化学的角度来看,这些碘(III)试剂可作为亲电子试剂,以有效地构建C–CF 3,X–CF 3(X =杂原子),C–R f(R f =全氟烷基),X–R f,C -N 3,C-CN,S-CN和C-X键。在某些情况下,必须使用路易斯酸或布朗斯台德酸来增加其亲电性。在这些转化中,碘(III)化合物以正式的“ CF 3+ ”,“ R f + ”,“ N 3 + ”,“ Ar + ”,“ CN + ”和“ X +”等价物。另一方面,I(III)试剂的一次电子还原为自由基世界打开了一扇门,这是该帐户的主题,该主题着重于高价碘(III)化合物(如Togni试剂,Zhdankin试剂)的自由基反应性,二芳基碘鎓盐,芳基碘鎓烷基化物,芳基(氰基)碘鎓三氟甲磺酸酯和芳基(全氟烷基)碘鎓三氟甲磺酸酯。以I(III)试剂开始的自由基生成也可以通过热或光介导的此类试剂中弱高价键的均质化而发生。此反应性可用于烷烃CH官能化。我们将致力于该领域的重要开拓性工作,但将主要侧重于我们小组在过去5年中进行的研究。
更新日期:2017-06-21
中文翻译:

自由基化学中的碘(III)试剂
在过去的30年中,高价碘(III)化合物的化学引起了人们极大的兴趣。高价碘(III)化合物由于其高亲电性而显示出宝贵的离子反应性,但作为碳和杂原子自由基生成的单电子氧化剂,也表现出自由基反应性。从离子化学的角度来看,这些碘(III)试剂可作为亲电子试剂,以有效地构建C–CF 3,X–CF 3(X =杂原子),C–R f(R f =全氟烷基),X–R f,C -N 3,C-CN,S-CN和C-X键。在某些情况下,必须使用路易斯酸或布朗斯台德酸来增加其亲电性。在这些转化中,碘(III)化合物以正式的“ CF 3+ ”,“ R f + ”,“ N 3 + ”,“ Ar + ”,“ CN + ”和“ X +”等价物。另一方面,I(III)试剂的一次电子还原为自由基世界打开了一扇门,这是该帐户的主题,该主题着重于高价碘(III)化合物(如Togni试剂,Zhdankin试剂)的自由基反应性,二芳基碘鎓盐,芳基碘鎓烷基化物,芳基(氰基)碘鎓三氟甲磺酸酯和芳基(全氟烷基)碘鎓三氟甲磺酸酯。以I(III)试剂开始的自由基生成也可以通过热或光介导的此类试剂中弱高价键的均质化而发生。此反应性可用于烷烃CH官能化。我们将致力于该领域的重要开拓性工作,但将主要侧重于我们小组在过去5年中进行的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号