当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Azapeptide Synthesis Methods for Expanding Side-Chain Diversity for Biomedical Applications
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-06-09 00:00:00 , DOI: 10.1021/acs.accounts.7b00114 Ramesh Chingle 1 , Caroline Proulx 1 , William D. Lubell 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-06-09 00:00:00 , DOI: 10.1021/acs.accounts.7b00114 Ramesh Chingle 1 , Caroline Proulx 1 , William D. Lubell 1
Affiliation
|
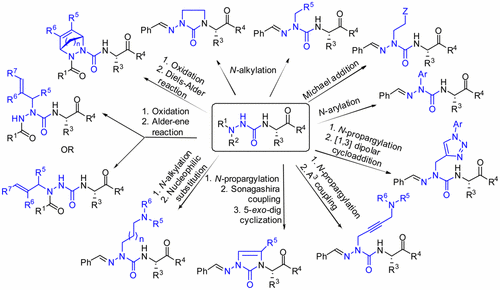
Mimicry of bioactive conformations is critical for peptide-based medicinal chemistry because such peptidomimetics may augment stability, enhance affinity, and increase specificity. Azapeptides are peptidomimetics in which the α-carbon(s) of one or more amino acid residues are substituted by nitrogen. The resulting semicarbazide analogues have been shown to reinforce β-turn conformation through the combination of lone pair–lone pair repulsion of the adjacent hydrazine nitrogen and urea planarity. Substitution of a semicarbazide for an amino amide residue in a peptide may retain biological activity and add benefits such as improved metabolic stability. The applications of azapeptides include receptor ligands, enzyme inhibitors, prodrugs, probes, and imaging agents. Moreover, azapeptides have proven therapeutic utility. For example, the aza-glycinamide analogue of the luteinizing hormone-releasing hormone analogue Zoladex is a potent long-acting agonist currently used in the clinic for the treatment of prostate and breast cancer. However, the use of azapeptides was hampered by tedious solution-phase synthetic routes for selective hydrazine functionalization. A remarkable stride to overcome this bottleneck was made in 2009 through the introduction of the submonomer procedure for azapeptide synthesis, which enabled addition of diverse side chains onto a common semicarbazone intermediate, providing a means to construct azapeptide libraries by solution- and solid-phase chemistry. In brief, aza residues are introduced into the peptide chain using the submonomer strategy by semicarbazone incorporation, deprotonation, N-alkylation, and orthogonal deprotection. Amino acylation of the resulting semicarbazide and elongation gives the desired azapeptide.
中文翻译:

用于扩大生物医学应用中侧链多样性的氮杂肽合成方法
模拟生物活性构象对于基于肽的药物化学至关重要,因为此类拟肽可以增加稳定性,增强亲和力并提高特异性。阿扎肽是拟肽,其中一个或多个氨基酸残基的α-碳被氮取代。结果表明,所得的氨基脲类似物可通过相邻肼氮的孤对-孤对排斥和尿素平面度的组合来增强β-转角构象。用氨基脲取代肽中的氨基酰胺残基可以保留生物学活性并增加诸如改善的代谢稳定性之类的益处。氮杂肽的应用包括受体配体,酶抑制剂,前药,探针和显像剂。此外,已经证明了氮杂肽具有治疗作用。例如,黄体生成激素释放激素类似物Zoladex的aza-glycinamide类似物是一种有效的长效激动剂,目前在临床上用于治疗前列腺癌和乳腺癌。然而,氮杂肽的使用被用于选择性肼功能化的繁琐的溶液相合成路线所阻碍。2009年,通过引入用于氮杂肽合成的亚单体方法,克服了这一瓶颈,迈出了显着的进步,该过程使得能够将各种侧链添加到常见的半脲中间体上,从而提供了一种通过溶液和固相化学方法构建氮杂肽文库的方法。简而言之,通过亚氨基甲酰胺掺入,去质子化,N-烷基化和正交去保护,使用亚单体策略将氮杂残基引入到肽链中。
更新日期:2017-06-09
中文翻译:

用于扩大生物医学应用中侧链多样性的氮杂肽合成方法
模拟生物活性构象对于基于肽的药物化学至关重要,因为此类拟肽可以增加稳定性,增强亲和力并提高特异性。阿扎肽是拟肽,其中一个或多个氨基酸残基的α-碳被氮取代。结果表明,所得的氨基脲类似物可通过相邻肼氮的孤对-孤对排斥和尿素平面度的组合来增强β-转角构象。用氨基脲取代肽中的氨基酰胺残基可以保留生物学活性并增加诸如改善的代谢稳定性之类的益处。氮杂肽的应用包括受体配体,酶抑制剂,前药,探针和显像剂。此外,已经证明了氮杂肽具有治疗作用。例如,黄体生成激素释放激素类似物Zoladex的aza-glycinamide类似物是一种有效的长效激动剂,目前在临床上用于治疗前列腺癌和乳腺癌。然而,氮杂肽的使用被用于选择性肼功能化的繁琐的溶液相合成路线所阻碍。2009年,通过引入用于氮杂肽合成的亚单体方法,克服了这一瓶颈,迈出了显着的进步,该过程使得能够将各种侧链添加到常见的半脲中间体上,从而提供了一种通过溶液和固相化学方法构建氮杂肽文库的方法。简而言之,通过亚氨基甲酰胺掺入,去质子化,N-烷基化和正交去保护,使用亚单体策略将氮杂残基引入到肽链中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号