当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A biosynthetically inspired route to substituted furans using the Appel reaction: total synthesis of the furan fatty acid F5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1039/c7cc03229c Robert J. Lee 1, 2, 3, 4, 5 , Martin R. Lindley 3, 4, 5, 6, 7 , Gareth J. Pritchard 1, 2, 3, 4, 5 , Marc C. Kimber 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-19 00:00:00 , DOI: 10.1039/c7cc03229c Robert J. Lee 1, 2, 3, 4, 5 , Martin R. Lindley 3, 4, 5, 6, 7 , Gareth J. Pritchard 1, 2, 3, 4, 5 , Marc C. Kimber 1, 2, 3, 4, 5
Affiliation
|
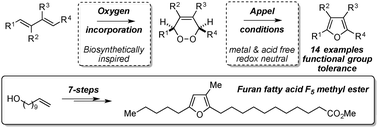
Appel reaction conditions have been harnessed to affect a mild biosynthetically inspired dehydration of endoperoxides to deliver multi-substituted electron rich furans. Unlike traditional dehydrative procedures, this method is metal and acid free, and can be achieved under redox neutral conditions. It is general for a range of aryl and alkyl substituted endoperoxides, and is functional group tolerant. Furthermore, this procedure has been used to deliver an effective total synthesis of the furan fatty acid (FFA) F5.
中文翻译:

利用Appel反应通过生物合成方法获得取代呋喃的途径:呋喃脂肪酸F 5的全合成
利用了Appel反应条件来影响内合成过氧化物的温和生物合成激发的脱水作用,以递送多取代的富电子呋喃。与传统的脱水程序不同,此方法不含金属和酸,可以在氧化还原中性条件下实现。通常适用于一定范围的芳基和烷基取代的内过氧化物,并且对官能团具有耐受性。此外,该程序已用于提供呋喃脂肪酸(FFA)F 5的有效全合成。
更新日期:2017-05-27
中文翻译:

利用Appel反应通过生物合成方法获得取代呋喃的途径:呋喃脂肪酸F 5的全合成
利用了Appel反应条件来影响内合成过氧化物的温和生物合成激发的脱水作用,以递送多取代的富电子呋喃。与传统的脱水程序不同,此方法不含金属和酸,可以在氧化还原中性条件下实现。通常适用于一定范围的芳基和烷基取代的内过氧化物,并且对官能团具有耐受性。此外,该程序已用于提供呋喃脂肪酸(FFA)F 5的有效全合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号