当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-Catalyzed bilateral cyclization of aldehydes with nitrosos toward unsymmetrical acridines proceeding with C–H functionalization enabled by a transient directing group
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-17 00:00:00 , DOI: 10.1039/c7cc03006a Weiming Hu 1, 2, 3, 4, 5 , Qingheng Zheng 1, 2, 3, 4, 5 , Song Sun 1, 2, 3, 4, 5 , Jiang Cheng 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-17 00:00:00 , DOI: 10.1039/c7cc03006a Weiming Hu 1, 2, 3, 4, 5 , Qingheng Zheng 1, 2, 3, 4, 5 , Song Sun 1, 2, 3, 4, 5 , Jiang Cheng 1, 2, 3, 4, 5
Affiliation
|
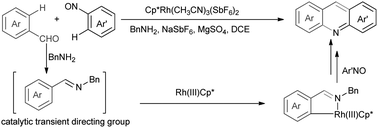
A Rh(III)-catalyzed bilateral cyclization was developed for the efficient construction of acridines proceeding with C–H functionalization whereby in situ formation and removal of an imino transient directing group in the presence of catalytic amount of BnNH2 are achieved. In this transformation, a sequential Rh(III)-catalyzed C–H amination, cyclization, and aromatization process was involved.
中文翻译:

Rh(III)催化的含亚硝基的醛向不对称bilateral啶的双侧环化反应,由一个瞬态导向基团进行C–H官能化
开发了Rh(III)催化的双环环化反应,可有效构建–啶并进行C–H官能化,从而在催化量的BnNH 2存在下原位形成和去除亚氨基瞬态导向基团。在此转化过程中,涉及到了连续的Rh(III)催化的CH–H胺化,环化和芳构化过程。
更新日期:2017-05-26
中文翻译:

Rh(III)催化的含亚硝基的醛向不对称bilateral啶的双侧环化反应,由一个瞬态导向基团进行C–H官能化
开发了Rh(III)催化的双环环化反应,可有效构建–啶并进行C–H官能化,从而在催化量的BnNH 2存在下原位形成和去除亚氨基瞬态导向基团。在此转化过程中,涉及到了连续的Rh(III)催化的CH–H胺化,环化和芳构化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号