当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iptycenes with an acridinone motif developed through [4+2] cycloaddition of tethered naphthalene and iminoquinone via a radical reaction
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-17 00:00:00 , DOI: 10.1039/c7cc03030d Selvam Raju,Pratheepkumar Annamalai,Pei-Ling Chen,Yi-Hung Liu,Shih-Ching Chuang
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-17 00:00:00 , DOI: 10.1039/c7cc03030d Selvam Raju,Pratheepkumar Annamalai,Pei-Ling Chen,Yi-Hung Liu,Shih-Ching Chuang
|
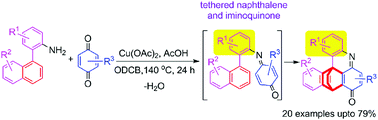
A new class of iptycenes was developed by combining 2-(naphthalen-1-yl)anilines and p-benzoquinones through copper(II)-mediated radical cyclisation. This unusual cyclisation reaction resulted in the robust and efficient syntheses of iptycenes with an acridinone motif. These iptycenes can be further transformed into planar acridinone heterocyclics through the Diels–Alder reaction.
中文翻译:

通过自由基反应通过拴系的萘和亚氨基醌的[4 + 2]环加成反应,开发出具有a啶酮基序的三萜
通过铜(II)介导的自由基环化作用,将2-(萘-1-基)苯胺和对-苯醌组合在一起,从而开发出一类新的三苯乙炔。这种不寻常的环化反应导致了带有an啶酮基序的iptycenes的稳健而高效的合成。通过狄尔斯-阿尔德反应,这些iptycenes可以进一步转化为平面的cri啶酮杂环。
更新日期:2017-05-25
中文翻译:

通过自由基反应通过拴系的萘和亚氨基醌的[4 + 2]环加成反应,开发出具有a啶酮基序的三萜
通过铜(II)介导的自由基环化作用,将2-(萘-1-基)苯胺和对-苯醌组合在一起,从而开发出一类新的三苯乙炔。这种不寻常的环化反应导致了带有an啶酮基序的iptycenes的稳健而高效的合成。通过狄尔斯-阿尔德反应,这些iptycenes可以进一步转化为平面的cri啶酮杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号