当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Dynamic Multisite Interactions between Two Intrinsically Disordered Proteins
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-24 08:25:28 , DOI: 10.1002/anie.201701883 Shaowen Wu 1 , Dongdong Wang 1 , Jin Liu 1 , Yitao Feng 1 , Jingwei Weng 1 , Yu Li 2 , Xin Gao 2 , Jianwei Liu 1 , Wenning Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-24 08:25:28 , DOI: 10.1002/anie.201701883 Shaowen Wu 1 , Dongdong Wang 1 , Jin Liu 1 , Yitao Feng 1 , Jingwei Weng 1 , Yu Li 2 , Xin Gao 2 , Jianwei Liu 1 , Wenning Wang 1
Affiliation
|
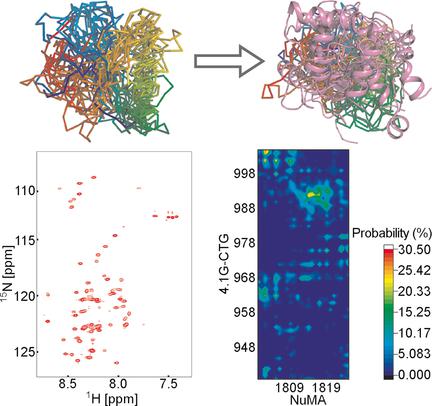
Protein interactions involving intrinsically disordered proteins (IDPs) comprise a variety of binding modes, from the well-characterized folding upon binding to dynamic fuzzy complexes. To date, most studies concern the binding of an IDP to a structured protein, while the interaction between two IDPs is poorly understood. In this study, NMR, smFRET, and molecular dynamics (MD) simulation are combined to characterize the interaction between two IDPs, the C-terminal domain (CTD) of protein 4.1G and the nuclear mitotic apparatus (NuMA) protein. It is revealed that CTD and NuMA form a fuzzy complex with remaining structural disorder. Multiple binding sites on both proteins were identified by molecular dynamics and mutagenesis studies. This study provides an atomic scenario in which two IDPs bearing multiple binding sites interact with each other in dynamic equilibrium. The combined approach employed here could be widely applicable for investigating IDPs and their dynamic interactions.
中文翻译:

两种内在无序蛋白之间的动态多位点相互作用
涉及内在无序蛋白(IDP)的蛋白相互作用包括多种结合模式,从结合到动态模糊复合物的特征明确的折叠开始。迄今为止,大多数研究都关注IDP与结构化蛋白质的结合,而对两个IDP之间的相互作用的了解却很少。在这项研究中,结合NMR,smFRET和分子动力学(MD)模拟来表征两个IDP,蛋白4.1G的C末端结构域(CTD)和核有丝分裂装置(NuMA)蛋白之间的相互作用。揭示了CTD和NuMA形成具有剩余结构紊乱的模糊复合物。通过分子动力学和诱变研究鉴定了两种蛋白质上的多个结合位点。这项研究提供了一个原子场景,其中两个带有多个结合位点的IDP在动态平衡中彼此相互作用。此处采用的组合方法可广泛应用于调查IDP及其动态交互。
更新日期:2017-05-25
中文翻译:

两种内在无序蛋白之间的动态多位点相互作用
涉及内在无序蛋白(IDP)的蛋白相互作用包括多种结合模式,从结合到动态模糊复合物的特征明确的折叠开始。迄今为止,大多数研究都关注IDP与结构化蛋白质的结合,而对两个IDP之间的相互作用的了解却很少。在这项研究中,结合NMR,smFRET和分子动力学(MD)模拟来表征两个IDP,蛋白4.1G的C末端结构域(CTD)和核有丝分裂装置(NuMA)蛋白之间的相互作用。揭示了CTD和NuMA形成具有剩余结构紊乱的模糊复合物。通过分子动力学和诱变研究鉴定了两种蛋白质上的多个结合位点。这项研究提供了一个原子场景,其中两个带有多个结合位点的IDP在动态平衡中彼此相互作用。此处采用的组合方法可广泛应用于调查IDP及其动态交互。











































 京公网安备 11010802027423号
京公网安备 11010802027423号