当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted 2-Acylaminocycloalkylthiophene-3-carboxylic Acid Arylamides as Inhibitors of the Calcium-Activated Chloride Channel Transmembrane Protein 16A (TMEM16A)
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00020 Eric C. Truong 1 , Puay W. Phuan 2 , Amanda L. Reggi 1 , Loretta Ferrera 3 , Luis J. V. Galietta 4 , Sarah E. Levy 1 , Alannah C. Moises 1 , Onur Cil 2 , Elena Diez-Cecilia 1 , Sujin Lee 2 , Alan S. Verkman 2 , Marc O. Anderson 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-11 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00020 Eric C. Truong 1 , Puay W. Phuan 2 , Amanda L. Reggi 1 , Loretta Ferrera 3 , Luis J. V. Galietta 4 , Sarah E. Levy 1 , Alannah C. Moises 1 , Onur Cil 2 , Elena Diez-Cecilia 1 , Sujin Lee 2 , Alan S. Verkman 2 , Marc O. Anderson 1
Affiliation
|
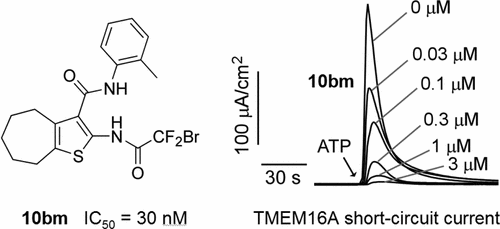
Transmembrane protein 16A (TMEM16A), also called anoctamin 1 (ANO1), is a calcium-activated chloride channel expressed widely mammalian cells, including epithelia, vascular smooth muscle tissue, electrically excitable cells, and some tumors. TMEM16A inhibitors have been proposed for treatment of disorders of epithelial fluid and mucus secretion, hypertension, asthma, and possibly cancer. Herein we report, by screening, the discovery of 2-acylaminocycloalkylthiophene-3-carboxylic acid arylamides (AACTs) as inhibitors of TMEM16A and analysis of 48 synthesized analogs (10ab–10bw) of the original AACT compound (10aa). Structure–activity studies indicated the importance of benzene substituted as 2- or 4-methyl, or 4-fluoro, and defined the significance of thiophene substituents and size of the cycloalkylthiophene core. The most potent compound (10bm), which contains an unusual bromodifluoroacetamide at the thiophene 2-position, had IC50 of ∼30 nM, ∼3.6-fold more potent than the most potent previously reported TMEM16A inhibitor 4 (Ani9), and >10-fold improved metabolic stability. Direct and reversible inhibition of TMEM16A by 10bm was demonstrated by patch-clamp analysis. AACTs may be useful as pharmacological tools to study TMEM16A function and as potential drug development candidates.
中文翻译:

取代的2-酰基氨基环烷基噻吩-3-羧酸芳基酰胺作为钙激活的氯离子通道跨膜蛋白16A(TMEM16A)的抑制剂
跨膜蛋白16A(TMEM16A),也称为八蛋氨酸1(ANO1),是一种钙激活的氯离子通道,广泛表达于哺乳动物细胞,包括上皮细胞,血管平滑肌组织,电兴奋性细胞和某些肿瘤。已经提出了TMEM16A抑制剂用于治疗上皮液和粘液分泌,高血压,哮喘和可能的癌症的疾病。本文我们报告,通过筛选,2- acylaminocycloalkylthiophene -3-羧酸芳基酰胺(AACTs)作为48个合成类似物(TMEM16A的抑制剂和分析发现10AB - 10bw)的原AACT化合物(10AA)。结构活性研究表明,苯被2-或4-甲基或4-氟取代的重要性,并定义了噻吩取代基的重要性和环烷基噻吩核的大小。最有效的化合物(10bm)在噻吩2位含有不寻常的溴二氟乙酰胺,IC 50约为30 nM,比以前报道的最有效的TMEM16A抑制剂4(Ani9)效力高约3.6倍,且> 10倍提高了代谢稳定性。通过膜片钳分析证实了10bm对TMEM16A的直接和可逆抑制。AACTs可用作研究TMEM16A功能的药理学工具和潜在的药物开发候选物。
更新日期:2017-05-25
中文翻译:

取代的2-酰基氨基环烷基噻吩-3-羧酸芳基酰胺作为钙激活的氯离子通道跨膜蛋白16A(TMEM16A)的抑制剂
跨膜蛋白16A(TMEM16A),也称为八蛋氨酸1(ANO1),是一种钙激活的氯离子通道,广泛表达于哺乳动物细胞,包括上皮细胞,血管平滑肌组织,电兴奋性细胞和某些肿瘤。已经提出了TMEM16A抑制剂用于治疗上皮液和粘液分泌,高血压,哮喘和可能的癌症的疾病。本文我们报告,通过筛选,2- acylaminocycloalkylthiophene -3-羧酸芳基酰胺(AACTs)作为48个合成类似物(TMEM16A的抑制剂和分析发现10AB - 10bw)的原AACT化合物(10AA)。结构活性研究表明,苯被2-或4-甲基或4-氟取代的重要性,并定义了噻吩取代基的重要性和环烷基噻吩核的大小。最有效的化合物(10bm)在噻吩2位含有不寻常的溴二氟乙酰胺,IC 50约为30 nM,比以前报道的最有效的TMEM16A抑制剂4(Ani9)效力高约3.6倍,且> 10倍提高了代谢稳定性。通过膜片钳分析证实了10bm对TMEM16A的直接和可逆抑制。AACTs可用作研究TMEM16A功能的药理学工具和潜在的药物开发候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号