当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
London Dispersion Enables the Shortest Intermolecular Hydrocarbon H···H Contact
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-13 00:00:00 , DOI: 10.1021/jacs.7b01879 Sören Rösel 1 , Henrik Quanz 1 , Christian Logemann 2 , Jonathan Becker 2 , Estelle Mossou 3, 4 , Laura Cañadillas-Delgado 3, 5 , Eike Caldeweyher 6 , Stefan Grimme 6 , Peter R. Schreiner 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-13 00:00:00 , DOI: 10.1021/jacs.7b01879 Sören Rösel 1 , Henrik Quanz 1 , Christian Logemann 2 , Jonathan Becker 2 , Estelle Mossou 3, 4 , Laura Cañadillas-Delgado 3, 5 , Eike Caldeweyher 6 , Stefan Grimme 6 , Peter R. Schreiner 1
Affiliation
|
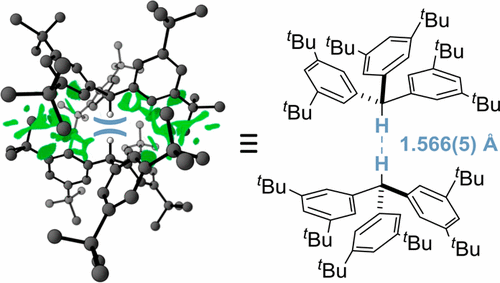
Neutron diffraction of tri(3,5-tert-butylphenyl)methane at 20 K reveals an intermolecular C–H···H–C distance of only 1.566(5) Å, which is the shortest reported to date. The compound crystallizes as a C3-symmetric dimer in an unusual head-to-head fashion. Quantum chemical computations of the solid state at the HSE-3c level of theory reproduce the structure and the close contact well (1.555 Å at 0 K) and emphasize the significance of packing effects; the gas-phase dimer structure at the same level shows a 1.634 Å C–H···H–C distance. Intermolecular London dispersion interactions between contacting tert-butyl substituents surrounding the central contact deliver the decisive energetic contributions to enable this remarkable bonding situation.
中文翻译:

伦敦分散体可实现最短的分子间烃H···H接触
三(3,5-叔丁基苯基)甲烷在20 K下的中子衍射显示,分子间C–H···H–C距离仅为1.566(5)Å,这是迄今为止报道的最短距离。该化合物以不寻常的头对头方式结晶为C 3对称二聚体。在HSE-3c理论水平上的固态量子化学计算再现了结构和紧密接触阱(在0 K下为1.555Å),并强调了堆积效应的重要性;相同水平的气相二聚体结构显示1.634ÅC–H··H–C距离。围绕中心接触的接触叔丁基取代基之间的分子间伦敦分散相互作用提供了决定性的能量贡献,使这种显着的键合情况成为可能。
更新日期:2017-05-24
中文翻译:

伦敦分散体可实现最短的分子间烃H···H接触
三(3,5-叔丁基苯基)甲烷在20 K下的中子衍射显示,分子间C–H···H–C距离仅为1.566(5)Å,这是迄今为止报道的最短距离。该化合物以不寻常的头对头方式结晶为C 3对称二聚体。在HSE-3c理论水平上的固态量子化学计算再现了结构和紧密接触阱(在0 K下为1.555Å),并强调了堆积效应的重要性;相同水平的气相二聚体结构显示1.634ÅC–H··H–C距离。围绕中心接触的接触叔丁基取代基之间的分子间伦敦分散相互作用提供了决定性的能量贡献,使这种显着的键合情况成为可能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号