当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size-dependent conformational change in halogen–π interaction: from benzene to graphene
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-15 00:00:00 , DOI: 10.1039/c7cc03116e Dong Yeon Kim 1, 2, 3, 4 , Jenica Marie L. Madridejos 2, 3, 4, 5 , Miran Ha 1, 2, 3, 4 , Jun-Hyeong Kim 2, 3, 4, 5 , David ChangMo Yang 2, 3, 4, 5 , Chunggi Baig 1, 2, 3, 4 , Kwang S. Kim 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-15 00:00:00 , DOI: 10.1039/c7cc03116e Dong Yeon Kim 1, 2, 3, 4 , Jenica Marie L. Madridejos 2, 3, 4, 5 , Miran Ha 1, 2, 3, 4 , Jun-Hyeong Kim 2, 3, 4, 5 , David ChangMo Yang 2, 3, 4, 5 , Chunggi Baig 1, 2, 3, 4 , Kwang S. Kim 2, 3, 4, 5
Affiliation
|
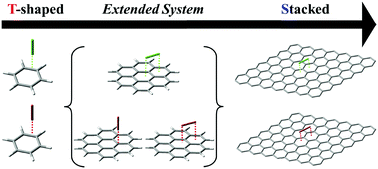
Diatomic halogen molecules X2 (X = Cl/Br) favor the edge-to-face conformation on benzene with significant electrostatic interaction via halogen bonding. In contrast, they favor the stacked conformation on graphene with negligible electrostatic interaction. As the aromatic ring expands, the inner facial side becomes almost electrostatically neutral. On coronene, the two conformations are compatible.
中文翻译:

卤素-π相互作用中尺寸依赖的构象变化:从苯到石墨烯
双原子卤素分子X 2(X = Cl / Br)有助于苯上的边对面构象,并通过卤素键具有显着的静电相互作用。相反,它们偏向于石墨烯上的堆叠构象,而静电相互作用可忽略不计。随着芳香环的膨胀,面部内侧几乎变成静电中性的。在异戊二烯上,这两个构象是相容的。
更新日期:2017-05-24
中文翻译:

卤素-π相互作用中尺寸依赖的构象变化:从苯到石墨烯
双原子卤素分子X 2(X = Cl / Br)有助于苯上的边对面构象,并通过卤素键具有显着的静电相互作用。相反,它们偏向于石墨烯上的堆叠构象,而静电相互作用可忽略不计。随着芳香环的膨胀,面部内侧几乎变成静电中性的。在异戊二烯上,这两个构象是相容的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号