当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Common Fibril Structures Imply Systemically Conserved Protein Misfolding Pathways In Vivo
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201701761 Karthikeyan Annamalai 1 , Falk Liberta 1 , Marie-Theres Vielberg 2 , William Close 1 , Hauke Lilie 3 , Karl-Heinz Gührs 4 , Angelika Schierhorn 5 , Rolf Koehler 6 , Andreas Schmidt 1 , Christian Haupt 1 , Ute Hegenbart 7 , Stefan Schönland 7 , Matthias Schmidt 1 , Michael Groll 2 , Marcus Fändrich 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 , DOI: 10.1002/anie.201701761 Karthikeyan Annamalai 1 , Falk Liberta 1 , Marie-Theres Vielberg 2 , William Close 1 , Hauke Lilie 3 , Karl-Heinz Gührs 4 , Angelika Schierhorn 5 , Rolf Koehler 6 , Andreas Schmidt 1 , Christian Haupt 1 , Ute Hegenbart 7 , Stefan Schönland 7 , Matthias Schmidt 1 , Michael Groll 2 , Marcus Fändrich 1
Affiliation
|
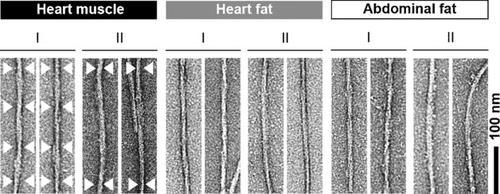
Systemic amyloidosis is caused by the misfolding of a circulating amyloid precursor protein and the deposition of amyloid fibrils in multiple organs. Chemical and biophysical analysis of amyloid fibrils from human AL and murine AA amyloidosis reveal the same fibril morphologies in different tissues or organs of one patient or diseased animal. The observed structural similarities concerned the fibril morphology, the fibril protein primary and secondary structures, the presence of post‐translational modifications and, in case of the AL fibrils, the partially folded characteristics of the polypeptide chain within the fibril. Our data imply for both analyzed forms of amyloidosis that the pathways of protein misfolding are systemically conserved; that is, they follow the same rules irrespective of where inside one body fibrils are formed or accumulated.
中文翻译:

常见的原纤维结构暗示体内系统保守的蛋白质错误折叠途径。
系统性淀粉样变性病是由循环的淀粉样蛋白前体蛋白的错误折叠和淀粉样蛋白原纤维在多个器官中的沉积引起的。来自人AL和鼠类AA淀粉样变性的淀粉样蛋白原纤维的化学和生物物理分析显示,在一名患者或患病动物的不同组织或器官中,原纤维形态相同。观察到的结构相似性涉及原纤维形态,原纤维蛋白的一级和二级结构,翻译后修饰的存在以及(对于AL原纤维而言)原纤维内多肽链的部分折叠特征。我们的数据表明,对于两种分析形式的淀粉样变性,蛋白质错误折叠的途径都是系统保守的。也就是说,它们遵循相同的规则,而不管在体内的原纤维形成或聚集的位置。
更新日期:2017-05-23
中文翻译:

常见的原纤维结构暗示体内系统保守的蛋白质错误折叠途径。
系统性淀粉样变性病是由循环的淀粉样蛋白前体蛋白的错误折叠和淀粉样蛋白原纤维在多个器官中的沉积引起的。来自人AL和鼠类AA淀粉样变性的淀粉样蛋白原纤维的化学和生物物理分析显示,在一名患者或患病动物的不同组织或器官中,原纤维形态相同。观察到的结构相似性涉及原纤维形态,原纤维蛋白的一级和二级结构,翻译后修饰的存在以及(对于AL原纤维而言)原纤维内多肽链的部分折叠特征。我们的数据表明,对于两种分析形式的淀粉样变性,蛋白质错误折叠的途径都是系统保守的。也就是说,它们遵循相同的规则,而不管在体内的原纤维形成或聚集的位置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号