当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Aplydactone by a Conformationally Controlled C−H Functionalization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 03:05:46 , DOI: 10.1002/anie.201703803 Chenguang Liu 1 , Renzhi Chen 1 , Yang Shen 1 , Zhanhao Liang 1 , Yuhui Hua 1 , Yandong Zhang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 03:05:46 , DOI: 10.1002/anie.201703803 Chenguang Liu 1 , Renzhi Chen 1 , Yang Shen 1 , Zhanhao Liang 1 , Yuhui Hua 1 , Yandong Zhang 1
Affiliation
|
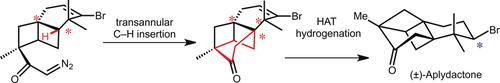
A concise, protecting-group-free total synthesis of the unusual brominated sesquiterpene aplydactone is described. Our synthesis features a [2+2] photocycloaddition, a Wolff ring contraction, an unusual remote C−H functionalization to establish the highly strained tetracyclic core, and a hydrogen-atom transfer (HAT) reaction to access the bromine-containing stereocenter. A finely tuned conformation of the α-diazoketone precursor is the key for the success of the late-stage transannular C−H insertion to deliver a bridged six-membered ring and a quaternary stereocenter (C6) between two quaternary carbon atoms (C1 and C7).
中文翻译:

通过构象控制的CH官能团全合成Aaplyactone
描述了一种简明的,无保护基团的不寻常的溴代倍半萜阿潘二酮的全合成方法。我们的合成具有[2 + 2]光环加成,Wolff环收缩,不寻常的远程CH功能化以建立高度应变的四环核,以及氢原子转移(HAT)反应以访问含溴的立体中心。α-二氮杂酮前体的微调构型是后期跨环CH插入成功的关键,以在两个四级碳原子(C1和C7)之间传递桥联的六元环和四级立体中心(C6) )。
更新日期:2017-05-24
中文翻译:

通过构象控制的CH官能团全合成Aaplyactone
描述了一种简明的,无保护基团的不寻常的溴代倍半萜阿潘二酮的全合成方法。我们的合成具有[2 + 2]光环加成,Wolff环收缩,不寻常的远程CH功能化以建立高度应变的四环核,以及氢原子转移(HAT)反应以访问含溴的立体中心。α-二氮杂酮前体的微调构型是后期跨环CH插入成功的关键,以在两个四级碳原子(C1和C7)之间传递桥联的六元环和四级立体中心(C6) )。











































 京公网安备 11010802027423号
京公网安备 11010802027423号