当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild, Redox-Neutral Formylation of Aryl Chlorides through the Photocatalytic Generation of Chlorine Radicals
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 02:31:10 , DOI: 10.1002/anie.201702079 Matthew K. Nielsen 1 , Benjamin J. Shields 1 , Junyi Liu 1 , Michael J. Williams 2 , Michael J. Zacuto 2 , Abigail G. Doyle 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-05-23 02:31:10 , DOI: 10.1002/anie.201702079 Matthew K. Nielsen 1 , Benjamin J. Shields 1 , Junyi Liu 1 , Michael J. Williams 2 , Michael J. Zacuto 2 , Abigail G. Doyle 1
Affiliation
|
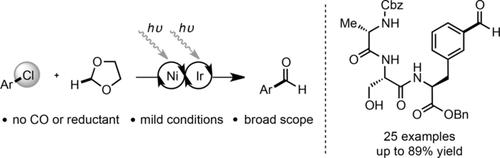
We report a redox-neutral formylation of aryl chlorides that proceeds through selective 2-functionalization of 1,3-dioxolane through nickel and photoredox catalysis. This scalable benchtop approach provides a distinct advantage over traditional reductive carbonylation in that no carbon monoxide, pressurized gas, or stoichiometric reductant is employed. The mild conditions give unprecedented scope from abundant and complex aryl chloride starting materials.
中文翻译:

通过光催化产生氯自由基,使芳基氯化物发生温和的氧化还原中性甲酰化反应
我们报告了通过镍和光氧化还原催化选择性的2-官能化1,3-二氧戊环进行的芳基氯化物的氧化还原中性甲酰化反应。这种可扩展的台式方法相对于传统的还原羰基化具有明显的优势,因为它不使用一氧化碳,加压气体或化学计量的还原剂。温和的条件为丰富而复杂的芳基氯原料提供了空前的应用范围。
更新日期:2017-05-24
中文翻译:

通过光催化产生氯自由基,使芳基氯化物发生温和的氧化还原中性甲酰化反应
我们报告了通过镍和光氧化还原催化选择性的2-官能化1,3-二氧戊环进行的芳基氯化物的氧化还原中性甲酰化反应。这种可扩展的台式方法相对于传统的还原羰基化具有明显的优势,因为它不使用一氧化碳,加压气体或化学计量的还原剂。温和的条件为丰富而复杂的芳基氯原料提供了空前的应用范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号