当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of DNA Nanostructures for High-Affinity Binding to Human Serum Albumin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1021/jacs.7b02917 Aurélie Lacroix 1 , Thomas G. W. Edwardson 1 , Mark A. Hancock 2 , Michael D. Dore 1 , Hanadi F. Sleiman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1021/jacs.7b02917 Aurélie Lacroix 1 , Thomas G. W. Edwardson 1 , Mark A. Hancock 2 , Michael D. Dore 1 , Hanadi F. Sleiman 1
Affiliation
|
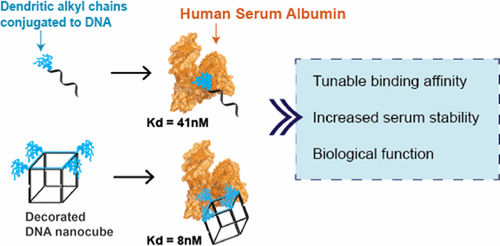
The development of nucleic acid therapeutics has been hampered by issues associated with their stability and in vivo delivery. To address these challenges, we describe a new strategy to engineer DNA structures with strong binding affinity to human serum albumin (HSA). HSA is the most abundant protein in the blood and has a long circulation half-life (19 days). It has been shown to hinder phagocytosis, is retained in tumors, and aids in cellular penetration. Indeed, HSA has already been successfully used for the delivery of small-molecule drugs and nanoparticles. We show that conjugating dendritic alkyl chains to DNA creates amphiphiles that exhibit high-affinity (Kd in low nanomolar range) binding to HSA. Notably, complexation with HSA did not hinder the activity of silencing oligonucleotides inside cells, and the degradation of DNA strands in serum was significantly slowed. We also show that, in a site-specific manner, altering the number and orientation of the amphiphilic ligand on a self-assembled DNA nanocube can modulate the affinity of the DNA cage to HSA. Moreover, the serum half-life of the amphiphile bound to the cage and the protein was shown to reach up to 22 hours, whereas unconjugated single-stranded DNA was degraded within minutes. Therefore, adding protein-specific binding domains to DNA nanostructures can be used to rationally control the interface between synthetic nanostructures and biological systems. A major challenge with nanoparticles delivery is the quick formation of a protein corona (i.e., protein adsorbed on the nanoparticle surface) upon injection to biological media. We foresee such DNA cage–protein complexes as new tools to study the role of this protein adsorption layer with important implications in the efficient delivery of RNAi therapeutics in vitro and in vivo.
中文翻译:

与人血清白蛋白高亲和力结合的DNA纳米结构的发展。
核酸治疗剂的开发已被与其稳定性和体内递送相关的问题所困扰。为了解决这些挑战,我们描述了一种新的策略来设计对人血清白蛋白(HSA)具有强结合亲和力的DNA结构。HSA是血液中最丰富的蛋白质,循环半衰期很长(19天)。已经显示出它可以阻止吞噬作用,保留在肿瘤中,并有助于细胞渗透。实际上,HSA已经成功用于递送小分子药物和纳米颗粒。我们显示,将树突状烷基链缀合至DNA会产生两亲物,这些两亲物表现出高亲和力(Kd在低纳摩尔范围内)与HSA的结合。值得注意的是,与HSA的复合作用不会阻碍细胞内沉默寡核苷酸的活性,并且血清中DNA链的降解显着减慢。我们还显示,以位点特异性的方式,改变自组装DNA纳米立方体上两亲性配体的数量和方向可以调节DNA笼对HSA的亲和力。此外,两亲分子结合笼子和蛋白的血清半衰期显示长达22小时,而未结合的单链DNA在数分钟内被降解。因此,在DNA纳米结构上添加蛋白质特异性结合域可用于合理控制合成纳米结构和生物系统之间的界面。纳米颗粒递送的主要挑战是注射到生物介质中后迅速形成蛋白质电晕(即吸附在纳米颗粒表面的蛋白质)。我们预见到这种DNA笼-蛋白复合物将成为研究该蛋白吸附层在有效输送RNAi治疗剂方面具有重要意义的新工具。体外和体内。
更新日期:2017-05-19
中文翻译:

与人血清白蛋白高亲和力结合的DNA纳米结构的发展。
核酸治疗剂的开发已被与其稳定性和体内递送相关的问题所困扰。为了解决这些挑战,我们描述了一种新的策略来设计对人血清白蛋白(HSA)具有强结合亲和力的DNA结构。HSA是血液中最丰富的蛋白质,循环半衰期很长(19天)。已经显示出它可以阻止吞噬作用,保留在肿瘤中,并有助于细胞渗透。实际上,HSA已经成功用于递送小分子药物和纳米颗粒。我们显示,将树突状烷基链缀合至DNA会产生两亲物,这些两亲物表现出高亲和力(Kd在低纳摩尔范围内)与HSA的结合。值得注意的是,与HSA的复合作用不会阻碍细胞内沉默寡核苷酸的活性,并且血清中DNA链的降解显着减慢。我们还显示,以位点特异性的方式,改变自组装DNA纳米立方体上两亲性配体的数量和方向可以调节DNA笼对HSA的亲和力。此外,两亲分子结合笼子和蛋白的血清半衰期显示长达22小时,而未结合的单链DNA在数分钟内被降解。因此,在DNA纳米结构上添加蛋白质特异性结合域可用于合理控制合成纳米结构和生物系统之间的界面。纳米颗粒递送的主要挑战是注射到生物介质中后迅速形成蛋白质电晕(即吸附在纳米颗粒表面的蛋白质)。我们预见到这种DNA笼-蛋白复合物将成为研究该蛋白吸附层在有效输送RNAi治疗剂方面具有重要意义的新工具。体外和体内。









































 京公网安备 11010802027423号
京公网安备 11010802027423号