当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Heterolytic Cleavage of the H–H Bond by Molybdenum Complexes: Controlling the Dynamics of Exchange Between Proton and Hydride
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-03 00:00:00 , DOI: 10.1021/jacs.7b03053 Shaoguang Zhang 1 , Aaron M. Appel 1 , R. Morris Bullock 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-03 00:00:00 , DOI: 10.1021/jacs.7b03053 Shaoguang Zhang 1 , Aaron M. Appel 1 , R. Morris Bullock 1
Affiliation
|
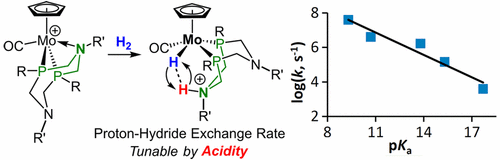
Controlling the heterolytic cleavage of the H–H bond of dihydrogen is critically important in catalytic hydrogenations and in the catalytic oxidation of H2. We show how the rate of reversible heterolytic cleavage of H2 can be controlled, spanning 4 orders of magnitude at 25 °C, from 2.1 × 103 s–1 to ≥107 s–1. Bifunctional Mo complexes, [CpMo(CO)(κ3-P2N2)]+ (P2N2 = 1,5-diaza-3,7-diphosphacyclooctane diphosphine ligand with alkyl/aryl groups on N and P), have been developed for heterolytic cleavage of H2 into a proton and a hydride, akin to frustrated Lewis pairs. The H–H bond cleavage is enabled by the basic amine in the second coordination sphere. The products of heterolytic cleavage of H2, Mo hydride complexes bearing protonated amines, [CpMo(H)(CO)(P2N2H)]+, were characterized by spectroscopic studies and by X-ray crystallography. Variable-temperature 1H, 15N, and 2-D 1H–1H ROESY NMR spectra indicated rapid exchange of the proton and hydride. The exchange rates are in the order [CpMo(H)(CO)(PPh2NPh2H)]+ > [CpMo(H)(CO)(PtBu2NPh2H)]+ > [CpMo(H)(CO)(PPh2NBn2H)]+ > [CpMo(H)(CO)(PtBu2NBn2H)]+ > [CpMo(H)(CO)(PtBu2NtBu2H)]+. The pKa values determined in acetonitrile range from 9.3 to 17.7 and show a linear correlation with the logarithm of the exchange rates. This correlation likely results from the exchange process involving key intermediates that differ by an intramolecular proton transfer. Specifically, the proton-hydride exchange appears to occur by formation of a molybdenum dihydride or dihydrogen complex, resulting from proton transfer from the pendant amine to the metal hydride. The exchange dynamics are controlled by the relative acidity of the [CpMo(H)(CO)(P2N2H)]+ and [CpMo(H2)(CO)(P2N2)]+ isomers, providing a design principle for controlling heterolytic cleavage of H2.
中文翻译:

钼配合物可逆地分解H-H键:控制质子与氢化物之间的交换动力学
在催化氢化和H 2催化氧化中,控制二氢H–H键的杂合裂解至关重要。我们将展示如何h的可逆异裂的速率2可以被控制,跨越4个数量级在25℃下,从2.1×10 3小号-1至≥10 7小号-1。双官能沫络合物,[合物CpMo(CO)(κ 3 -P 2 Ñ 2)] +(P 2 Ñ 2 = 1,5-二氮杂-3,7-二diphosphacyclooctane二膦配体与烷基/芳基上的N和P基团),已开发用于H 2的异源裂解变成质子和氢化物,类似于沮丧的路易斯对。在第二个配位域中,碱性胺可实现H–H键的裂解。通过光谱研究和X射线晶体学表征了H 2,带有质子化胺的氢化钼配合物[CpMo(H)(CO)(P 2 N 2 H)] +的氢解裂解产物。温度可变的1 H,15 N和2-D 1 H - 1 H ROESY NMR光谱表明质子和氢化物的快速交换。汇率按[CpMo(H)(CO)(P Ph 2 N Ph 2H)] + > [CpMo(H)(CO)(P t Bu 2 N Ph 2 H)] + > [CpMo(H)(CO)(P Ph 2 N Bn 2 H)] + > [CpMo(H)(CO)(P t Bu 2 N Bn 2 H)] + > [CpMo(H)(CO)(P t Bu 2 N t Bu 2 H)] +。在P ķ一乙腈中测定的值介于9.3至17.7之间,并且与汇率的对数呈线性相关。这种相关性可能是由于涉及分子中间体质子转移而不同的关键中间体的交换过程所致。具体地说,质子-氢化物交换似乎是由于质子从侧基胺转移到金属氢化物而形成的二氢化钼或二氢络合物而发生的。交换动力学受[CpMo(H)(CO)(P 2 N 2 H)] +和[CpMo(H 2)(CO)(P 2 N 2)] +的相对酸度控制。异构体,提供了控制H 2的异源裂解的设计原理。
更新日期:2017-05-19
中文翻译:

钼配合物可逆地分解H-H键:控制质子与氢化物之间的交换动力学
在催化氢化和H 2催化氧化中,控制二氢H–H键的杂合裂解至关重要。我们将展示如何h的可逆异裂的速率2可以被控制,跨越4个数量级在25℃下,从2.1×10 3小号-1至≥10 7小号-1。双官能沫络合物,[合物CpMo(CO)(κ 3 -P 2 Ñ 2)] +(P 2 Ñ 2 = 1,5-二氮杂-3,7-二diphosphacyclooctane二膦配体与烷基/芳基上的N和P基团),已开发用于H 2的异源裂解变成质子和氢化物,类似于沮丧的路易斯对。在第二个配位域中,碱性胺可实现H–H键的裂解。通过光谱研究和X射线晶体学表征了H 2,带有质子化胺的氢化钼配合物[CpMo(H)(CO)(P 2 N 2 H)] +的氢解裂解产物。温度可变的1 H,15 N和2-D 1 H - 1 H ROESY NMR光谱表明质子和氢化物的快速交换。汇率按[CpMo(H)(CO)(P Ph 2 N Ph 2H)] + > [CpMo(H)(CO)(P t Bu 2 N Ph 2 H)] + > [CpMo(H)(CO)(P Ph 2 N Bn 2 H)] + > [CpMo(H)(CO)(P t Bu 2 N Bn 2 H)] + > [CpMo(H)(CO)(P t Bu 2 N t Bu 2 H)] +。在P ķ一乙腈中测定的值介于9.3至17.7之间,并且与汇率的对数呈线性相关。这种相关性可能是由于涉及分子中间体质子转移而不同的关键中间体的交换过程所致。具体地说,质子-氢化物交换似乎是由于质子从侧基胺转移到金属氢化物而形成的二氢化钼或二氢络合物而发生的。交换动力学受[CpMo(H)(CO)(P 2 N 2 H)] +和[CpMo(H 2)(CO)(P 2 N 2)] +的相对酸度控制。异构体,提供了控制H 2的异源裂解的设计原理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号