当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold-catalyzed oxidative hydroacylation reactions of α-iminoalkynes with aldehydes and O2
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1039/c7cc03421k Yu-Chen Hsu 1, 2, 3, 4 , Jing-He Lai 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2017-05-12 00:00:00 , DOI: 10.1039/c7cc03421k Yu-Chen Hsu 1, 2, 3, 4 , Jing-He Lai 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Affiliation
|
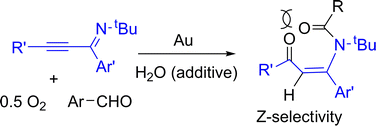
Gold-catalyzed aerobic oxidations of α-iminoalkynes with aryl aldehydes led to oxidative 1,3-hydroacyclation reactions, yielding high Z-selectivity. We performed 2H- and 18O-labeling experiments to confirm the role of water as an oxygen donor and O2 as the main oxidant. The Z-selectivity arises from an efficient conjugation between the enone and its aryl substituent. We postulate an initial [4+2]-annulation of α-iminoalkynes with aldehydes to form a six-membered oxonium species that is attacked by water, followed by the O2 oxidation of a key intermediate.
中文翻译:

金催化的α-亚氨基炔烃与醛和O 2的氧化加氢酰化反应
金催化的α-亚氨基炔烃与芳基醛的需氧氧化导致氧化的1,3-氢环化反应,产生高Z选择性。我们进行了2次H-标记和18次O-标记实验,以确认水作为氧气供体和O 2作为主要氧化剂的作用。所述ž -选择性起因于烯酮和其芳基取代基之间的有效偶联。我们假设α-亚氨基炔烃与醛的初始[4 + 2]环化反应形成六元的氧鎓类物质,该类物质受到水的攻击,然后被关键中间体的O 2氧化。
更新日期:2017-05-19
中文翻译:

金催化的α-亚氨基炔烃与醛和O 2的氧化加氢酰化反应
金催化的α-亚氨基炔烃与芳基醛的需氧氧化导致氧化的1,3-氢环化反应,产生高Z选择性。我们进行了2次H-标记和18次O-标记实验,以确认水作为氧气供体和O 2作为主要氧化剂的作用。所述ž -选择性起因于烯酮和其芳基取代基之间的有效偶联。我们假设α-亚氨基炔烃与醛的初始[4 + 2]环化反应形成六元的氧鎓类物质,该类物质受到水的攻击,然后被关键中间体的O 2氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号