当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
12,13-Aziridinyl Epothilones. Stereoselective Synthesis of Trisubstituted Olefinic Bonds from Methyl Ketones and Heteroaromatic Phosphonates and Design, Synthesis, and Biological Evaluation of Potent Antitumor Agents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-17 , DOI: 10.1021/jacs.7b02655 K. C. Nicolaou 1 , Derek Rhoades 1 , Yanping Wang 1 , Ruoli Bai 2 , Ernest Hamel 2 , Monette Aujay 3 , Joseph Sandoval 3 , Julia Gavrilyuk 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-17 , DOI: 10.1021/jacs.7b02655 K. C. Nicolaou 1 , Derek Rhoades 1 , Yanping Wang 1 , Ruoli Bai 2 , Ernest Hamel 2 , Monette Aujay 3 , Joseph Sandoval 3 , Julia Gavrilyuk 3
Affiliation
|
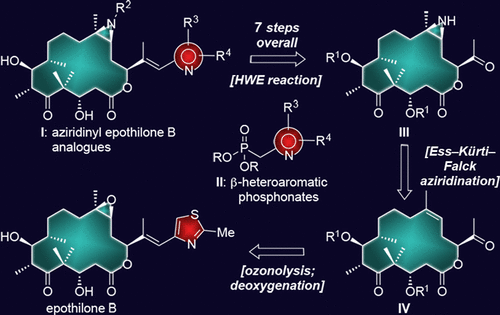
The synthesis and biological evaluation of a series of 12,13-aziridinyl epothilone B analogues is described. These compounds were accessed by a practical, general process that involved a 12,13-olefinic methyl ketone as a starting material obtained by ozonolytic cleavage of epothilone B followed by tungsten-induced deoxygenation of the epoxide moiety. The attachment of the aziridine structural motif was achieved by application of the Ess-Kürti-Falck aziridination, while the heterocyclic side chains were introduced via stereoselective phosphonate-based olefinations. In order to ensure high (E) selectivities for the latter reaction for electron-rich heterocycles, it became necessary to develop and apply an unprecedented modification of the venerable Horner-Wadsworth-Emmons reaction, employing 2-fluoroethoxyphosphonates that may prove to be of general value in organic synthesis. These studies resulted in the discovery of some of the most potent epothilones reported to date. Equipped with functional groups to accommodate modern drug delivery technologies, some of these compounds exhibited picomolar potencies that qualify them as payloads for antibody drug conjugates (ADCs), while a number of them revealed impressive activities against drug resistant human cancer cells, making them desirable for potential medical applications.
中文翻译:

12,13-氮丙啶基埃坡霉素。甲基酮和杂芳族膦酸酯的三取代烯烃键的立体选择性合成以及有效抗肿瘤剂的设计、合成和生物学评价
描述了一系列 12,13-氮丙啶埃坡霉素 B 类似物的合成和生物学评价。这些化合物是通过一种实用的通用方法获得的,该方法涉及以 12,13-烯属甲基酮作为起始材料,通过臭氧分解埃坡霉素 B 然后钨诱导的环氧化物部分脱氧获得。氮丙啶结构基序的连接是通过应用 Ess-Kürti-Falck 氮丙啶化来实现的,而杂环侧链则是通过基于立体选择性膦酸酯的烯化作用引入的。为了确保后一种富电子杂环反应的高 (E) 选择性,有必要对古老的 Horner-Wadsworth-Emmons 反应进行前所未有的改进,使用 2-氟乙氧基膦酸酯,可能被证明在有机合成中具有普遍价值。这些研究导致发现了迄今为止报道的一些最有效的埃坡霉素。配备了适应现代药物递送技术的官能团,这些化合物中的一些表现出皮摩尔的效力,使它们成为抗体药物偶联物 (ADC) 的有效载荷,而其中一些化合物显示出令人印象深刻的抗耐药性人类癌细胞的活性,使其成为理想潜在的医疗应用。
更新日期:2017-05-17
中文翻译:

12,13-氮丙啶基埃坡霉素。甲基酮和杂芳族膦酸酯的三取代烯烃键的立体选择性合成以及有效抗肿瘤剂的设计、合成和生物学评价
描述了一系列 12,13-氮丙啶埃坡霉素 B 类似物的合成和生物学评价。这些化合物是通过一种实用的通用方法获得的,该方法涉及以 12,13-烯属甲基酮作为起始材料,通过臭氧分解埃坡霉素 B 然后钨诱导的环氧化物部分脱氧获得。氮丙啶结构基序的连接是通过应用 Ess-Kürti-Falck 氮丙啶化来实现的,而杂环侧链则是通过基于立体选择性膦酸酯的烯化作用引入的。为了确保后一种富电子杂环反应的高 (E) 选择性,有必要对古老的 Horner-Wadsworth-Emmons 反应进行前所未有的改进,使用 2-氟乙氧基膦酸酯,可能被证明在有机合成中具有普遍价值。这些研究导致发现了迄今为止报道的一些最有效的埃坡霉素。配备了适应现代药物递送技术的官能团,这些化合物中的一些表现出皮摩尔的效力,使它们成为抗体药物偶联物 (ADC) 的有效载荷,而其中一些化合物显示出令人印象深刻的抗耐药性人类癌细胞的活性,使其成为理想潜在的医疗应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号