当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct 11CN-Labeling of Unprotected Peptides via Palladium-Mediated Sequential Cross-Coupling Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-15 00:00:00 , DOI: 10.1021/jacs.7b02761 Wenjun Zhao 1, 2 , Hong Geun Lee 3 , Stephen L. Buchwald 3 , Jacob M. Hooker 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-05-15 00:00:00 , DOI: 10.1021/jacs.7b02761 Wenjun Zhao 1, 2 , Hong Geun Lee 3 , Stephen L. Buchwald 3 , Jacob M. Hooker 1, 2
Affiliation
|
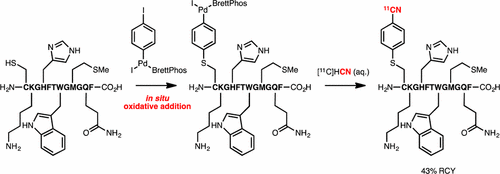
A practical procedure for 11CN-labeling of unprotected peptides has been developed. The method was shown to be highly chemoselective for cysteine over other potentially nucleophilic residues, and the radiolabeled products were synthesized and purified in less than 15 min. Appropriate for biomedical applications, the method could be used on an extremely small scale (20 nmol) with a high radiochemical yield. The success of the protocol stems from the use of a Pd-reagent based on a dihaloarene, which enables direct “nucleophile–nucleophile” coupling of the peptide and [11C]cyanide by temporal separation of nucleophile addition.
中文翻译:

通过钯介导的顺序交叉偶联反应对未保护肽进行直接11 CN标记
已经开发了用于11 CN标记未保护肽的实用程序。结果表明,该方法对半胱氨酸的化学选择性高于其他潜在的亲核残基,并且可以在不到15分钟的时间内合成并纯化放射性标记的产物。适用于生物医学应用,该方法可用于极小规模(20 nmol)且放射化学收率高。该协议的成功源于使用基于二卤代芳烃的Pd试剂,该试剂可通过暂时分离亲核试剂添加而使肽与[ 11 C]氰化物直接“亲核-亲核”偶联。
更新日期:2017-05-18
中文翻译:

通过钯介导的顺序交叉偶联反应对未保护肽进行直接11 CN标记
已经开发了用于11 CN标记未保护肽的实用程序。结果表明,该方法对半胱氨酸的化学选择性高于其他潜在的亲核残基,并且可以在不到15分钟的时间内合成并纯化放射性标记的产物。适用于生物医学应用,该方法可用于极小规模(20 nmol)且放射化学收率高。该协议的成功源于使用基于二卤代芳烃的Pd试剂,该试剂可通过暂时分离亲核试剂添加而使肽与[ 11 C]氰化物直接“亲核-亲核”偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号