当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tolvaptan-Type Vasopressin Receptor Ligands: Important Role of Axial Chirality in the Active Form
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00422 Hidetsugu Tabata 1 , Tetsuya Yoneda 1 , Tetsuta Oshitari 1 , Hideyo Takahashi 1 , Hideaki Natsugari 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00422 Hidetsugu Tabata 1 , Tetsuya Yoneda 1 , Tetsuta Oshitari 1 , Hideyo Takahashi 1 , Hideaki Natsugari 1
Affiliation
|
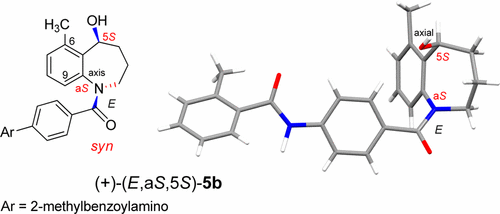
The anti and syn isomers of tolvaptan-type compounds, N-benzoyl-5-hydroxy-1-benzazepines (5a–c), were prepared in a stereocontrolled manner by biasing the conformation with a methyl group at C9 and C6, respectively, and the enantiomeric forms were separated. Examination of the affinity at the human vasopressin receptors revealed that the axial chirality (aS) plays a more important role than the central chirality at C5 in receptor recognition, and the most preferable form was shown to be (E,aS,5S).
中文翻译:

托伐普坦型加压素受体配体:活性形式的轴向手性的重要作用。
所述抗和顺式的托伐普坦类化合物的异构体,ñ -苯甲酰基-5-羟基-1-苯并吖庚因(5A - Ç),是在一个立体控制的方式通过偏压与在分别C9和C6,甲基构象制备,并且对映体形式被分离。对人血管加压素受体的亲和力研究表明,在受体识别中,轴向手性(a S)比C 5的中心手性更重要,并且最优选的形式为(E,a S,5 S)。
更新日期:2017-05-17
中文翻译:

托伐普坦型加压素受体配体:活性形式的轴向手性的重要作用。
所述抗和顺式的托伐普坦类化合物的异构体,ñ -苯甲酰基-5-羟基-1-苯并吖庚因(5A - Ç),是在一个立体控制的方式通过偏压与在分别C9和C6,甲基构象制备,并且对映体形式被分离。对人血管加压素受体的亲和力研究表明,在受体识别中,轴向手性(a S)比C 5的中心手性更重要,并且最优选的形式为(E,a S,5 S)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号