当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diindolylmethane Derivatives: Potent Agonists of the Immunostimulatory Orphan G Protein-Coupled Receptor GPR84
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01593 Thanigaimalai Pillaiyar 1 , Meryem Köse 1 , Katharina Sylvester 1 , Heike Weighardt 2 , Dominik Thimm 1 , Gleice Borges 1 , Irmgard Förster 2 , Ivar von Kügelgen 3 , Christa E. Müller 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01593 Thanigaimalai Pillaiyar 1 , Meryem Köse 1 , Katharina Sylvester 1 , Heike Weighardt 2 , Dominik Thimm 1 , Gleice Borges 1 , Irmgard Förster 2 , Ivar von Kügelgen 3 , Christa E. Müller 1
Affiliation
|
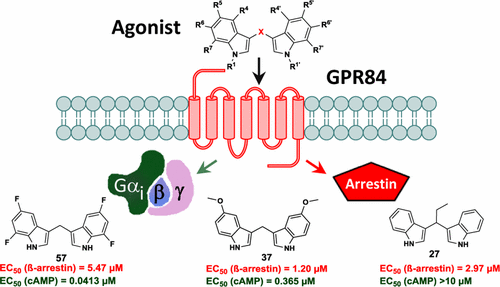
The Gi protein-coupled receptor GPR84, which is activated by (hydroxy)fatty acids, is highly expressed on immune cells. Recently, 3,3′-diindolylmethane was identified as a heterocyclic, nonlipid-like GPR84 agonist. We synthesized a broad range of diindolylmethane derivatives by condensation of indoles with formaldehyde in water under microwave irradiation. The products were evaluated at the human GPR84 in cAMP and β-arrestin assays. Structure–activity relationships (SARs) were steep. 3,3′-Diindolylmethanes bearing small lipophilic residues at the 5- and/or 7-position of the indole rings displayed the highest activity in cAMP assays, the most potent agonists being di(5-fluoro-1H-indole-3-yl)methane (38, PSB-15160, EC50 80.0 nM) and di(5,7-difluoro-1H-indole-3-yl)methane (57, PSB-16671, EC50 41.3 nM). In β-arrestin assays, SARs were different, indicating biased agonism. The new compounds were selective versus related fatty acid receptors and the arylhydrocarbon receptor. Selected compounds were further investigated and found to display an ago-allosteric mechanism of action and increased stability in comparison to the lead structure.
中文翻译:

Diindolylmethane衍生物:免疫刺激性孤儿G蛋白偶联受体GPR84的强效激动剂。
对于g我蛋白偶联受体GPR84,它是由(羟基)脂肪酸,激活的免疫细胞上高表达。最近,3,3'-二吲哚基甲烷被鉴定为杂环类非脂质GPR84激动剂。我们在微波辐射下通过吲哚与甲醛在水中的缩合反应合成了多种二吲哚甲烷衍生物。在人GPR84上的cAMP和β-arrestin检测中对产品进行了评估。构效关系(SARs)很陡。在cAMP分析中,在吲哚环的5和/或7位带有小的亲脂性残基的3,3'-二吲哚基甲烷显示出最高的活性,最有效的激动剂是di(5-fluoro-1 H -indole-3-甲烷)(38,PSB-15160,EC 5080.0 nM)和二(5,7-二氟-1 H-吲哚-3-基)甲烷(57,PSB-16671,EC 50 41.3 nM)。在β-arrestin检测中,SAR有所不同,表明激动作用存在偏倚。新化合物对相关的脂肪酸受体和芳基烃受体具有选择性。进一步研究了选定的化合物,发现与前导结构相比,该化合物表现出较早的变构作用机理和更高的稳定性。
更新日期:2017-05-01
中文翻译:

Diindolylmethane衍生物:免疫刺激性孤儿G蛋白偶联受体GPR84的强效激动剂。
对于g我蛋白偶联受体GPR84,它是由(羟基)脂肪酸,激活的免疫细胞上高表达。最近,3,3'-二吲哚基甲烷被鉴定为杂环类非脂质GPR84激动剂。我们在微波辐射下通过吲哚与甲醛在水中的缩合反应合成了多种二吲哚甲烷衍生物。在人GPR84上的cAMP和β-arrestin检测中对产品进行了评估。构效关系(SARs)很陡。在cAMP分析中,在吲哚环的5和/或7位带有小的亲脂性残基的3,3'-二吲哚基甲烷显示出最高的活性,最有效的激动剂是di(5-fluoro-1 H -indole-3-甲烷)(38,PSB-15160,EC 5080.0 nM)和二(5,7-二氟-1 H-吲哚-3-基)甲烷(57,PSB-16671,EC 50 41.3 nM)。在β-arrestin检测中,SAR有所不同,表明激动作用存在偏倚。新化合物对相关的脂肪酸受体和芳基烃受体具有选择性。进一步研究了选定的化合物,发现与前导结构相比,该化合物表现出较早的变构作用机理和更高的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号