当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid–Base Catalysis in Glycosidations: A Nature Derived Alternative to the Generally Employed Methodology
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-04-25 00:00:00 , DOI: 10.1021/acs.accounts.6b00518 Peng Peng 1 , Richard R. Schmidt 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-04-25 00:00:00 , DOI: 10.1021/acs.accounts.6b00518 Peng Peng 1 , Richard R. Schmidt 2
Affiliation
|
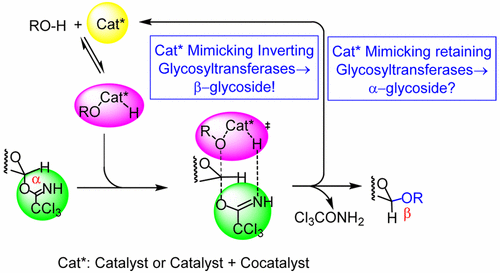
Inverting glycosyltransferases enforce in the active site an intramolecular, acid–base catalyzed glycosidation that, due to proximity of the donor anomeric carbon and the acceptor hydroxyl group, follows an SN2-type reaction. Spacers, tethering donor and acceptor via nonreacting functional groups, led in intramolecular glycosidations to excellent yields and, independent of the donor anomeric configuration, to either the α- or the β-anomer. The requirement of a demanding protecting group pattern confines the application of this efficient method. Only the method where the 2-hydroxyl group of a mannopyranosyl donor is tethered via an acetal spacer to the reacting acceptor functional group is used for β-mannopyranoside synthesis. The most elegant method, tethering donor and acceptor covalently to the spacer via the leaving group and the reacting functional group, was so far not as efficient as hoped. This method is very efficient when donor and acceptor are temporarily assembled through a hydrogen-bond facilitating a stretched hexagon-like transition state. This follows from the stereoselective O-glucopyranosyl trichloroacetimidate transformation into O-glucopyranosyl phosphate with dibenzyl phosphoric acid as acceptor that can be regarded as A═B–C–H acceptor type. Generalizing this concept to the use of alcohols as acceptors requires reversible generation of an A–B–C–H adduct where A–H represents the acceptor (RO–H) and B═C a catalyst that has to fulfill several criteria. Among these criteria are low affinity to nitrogen, avoiding glycosyl donor activation in the absence of acceptor, and high affinity to oxygen in order to generate the A–B–C–H adduct with increased proton acidity. Thus, hydrogen-bond mediated self-assembly of donor and acceptor and concomitant donor activation via a transition state is available, which enforces an acid–base catalyzed SN2-type reaction. It could be shown that PhBF2, Ph2BF, and PhSiF3 are such catalysts that fulfill the desired four functions: reversible adduct formation with the acceptor, hydrogen-bond mediated tethering of this adduct with the donor, and acid- and base-catalysis of the glycosidation. Also Lewis acidic metal salts, particularly the dimeric gold(III) chloride, turned out to exhibit excellent B═C type catalyst properties. Worth mentioning in this context is the ability of gold(III) chloride to regioselectively activate diols.
中文翻译:

糖基化中的酸基催化:自然界替代一般采用的方法
反向糖基转移酶在活性位点强制分子内酸基催化的糖苷化,由于供体异头碳和受体羟基的接近,S N2型反应。经由非反应性官能团束缚供体和受体的间隔物导致分子内糖苷化产生优异的产率,并且与供体端基异构体构型无关地导致α-或β-异头物。苛刻的保护基团图案的要求限制了这种有效方法的应用。仅将甘露吡喃糖基供体的2-羟基经由缩醛间隔子束缚至反应性受体官能团的方法用于β-甘露吡喃糖苷的合成。迄今为止,最优雅的方法是通过离去基团和反应的官能团将供体和受体共价连接到间隔基上,但效率不如人们所希望的那样。当供体和受体通过氢键临时组装以促进伸展的六角形过渡态时,此方法非常有效。ø吡喃葡萄糖基三氯乙酰亚胺酯转化为ö吡喃葡萄糖基磷酸二苄基与磷酸作为受体,可以被视为A═B-C-H受体类型。将这一概念推广到使用醇作为受体,需要可逆生成A–B–C–H加合物,其中A–H代表受体(RO–H),B═C是必须满足多个标准的催化剂。这些标准包括对氮的低亲和力,避免在没有受体的情况下糖基供体的活化以及对氧的高亲和力,以产生质子酸度增加的A–B–C–H加合物。因此,可利用氢键介导的供体和受体的自组装以及通过过渡态的伴随供体活化,从而增强了酸碱催化的S N。2型反应。可以证明,PhBF 2,Ph 2 BF和PhSiF 3是满足所需四个功能的催化剂:与受体的可逆加合物形成,该加合物与供体的氢键介导的束缚以及酸和碱糖苷化的催化。还发现路易斯酸性金属盐,特别是二聚氯化金(III),表现出优异的B═C型催化剂性能。在这方面值得一提的是氯化金(III)区域选择性活化二醇的能力。
更新日期:2017-04-25
中文翻译:

糖基化中的酸基催化:自然界替代一般采用的方法
反向糖基转移酶在活性位点强制分子内酸基催化的糖苷化,由于供体异头碳和受体羟基的接近,S N2型反应。经由非反应性官能团束缚供体和受体的间隔物导致分子内糖苷化产生优异的产率,并且与供体端基异构体构型无关地导致α-或β-异头物。苛刻的保护基团图案的要求限制了这种有效方法的应用。仅将甘露吡喃糖基供体的2-羟基经由缩醛间隔子束缚至反应性受体官能团的方法用于β-甘露吡喃糖苷的合成。迄今为止,最优雅的方法是通过离去基团和反应的官能团将供体和受体共价连接到间隔基上,但效率不如人们所希望的那样。当供体和受体通过氢键临时组装以促进伸展的六角形过渡态时,此方法非常有效。ø吡喃葡萄糖基三氯乙酰亚胺酯转化为ö吡喃葡萄糖基磷酸二苄基与磷酸作为受体,可以被视为A═B-C-H受体类型。将这一概念推广到使用醇作为受体,需要可逆生成A–B–C–H加合物,其中A–H代表受体(RO–H),B═C是必须满足多个标准的催化剂。这些标准包括对氮的低亲和力,避免在没有受体的情况下糖基供体的活化以及对氧的高亲和力,以产生质子酸度增加的A–B–C–H加合物。因此,可利用氢键介导的供体和受体的自组装以及通过过渡态的伴随供体活化,从而增强了酸碱催化的S N。2型反应。可以证明,PhBF 2,Ph 2 BF和PhSiF 3是满足所需四个功能的催化剂:与受体的可逆加合物形成,该加合物与供体的氢键介导的束缚以及酸和碱糖苷化的催化。还发现路易斯酸性金属盐,特别是二聚氯化金(III),表现出优异的B═C型催化剂性能。在这方面值得一提的是氯化金(III)区域选择性活化二醇的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号