当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Asymmetric Hydrogenation of Unsaturated Carboxylic Acids
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-04-04 00:00:00 , DOI: 10.1021/acs.accounts.7b00007 Shou-Fei Zhu 1 , Qi-Lin Zhou 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-04-04 00:00:00 , DOI: 10.1021/acs.accounts.7b00007 Shou-Fei Zhu 1 , Qi-Lin Zhou 1
Affiliation
|
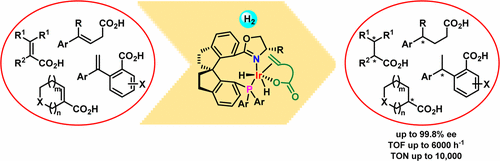
Chiral carboxylic acid moieties are widely found in pharmaceuticals, agrochemicals, flavors, fragrances, and health supplements. Although they can be synthesized straightforwardly by transition-metal-catalyzed enantioselective hydrogenation of unsaturated carboxylic acids, because the existing chiral catalysts have various disadvantages, the development of new chiral catalysts with high activity and enantioselectivity is an important, long-standing challenge. Ruthenium complexes with chiral diphosphine ligands and rhodium complexes with chiral monodentate or bidentate phosphorus ligands have been the predominant catalysts for asymmetric hydrogenation of unsaturated acids. However, the efficiency of these catalysts is highly substrate-dependent, and most of the reported catalysts require a high loading, high hydrogen pressure, or long reaction time for satisfactory results.
中文翻译:

铱催化不饱和羧酸的不对称加氢
手性羧酸部分广泛用于药物,农用化学品,调味剂,香料和保健补品中。尽管它们可以通过过渡金属催化的不饱和羧酸的对映选择性氢化直接合成,但是由于现有的手性催化剂具有各种缺点,因此开发具有高活性和对映选择性的新型手性催化剂是一项重要的长期挑战。具有手性二膦配体的钌配合物和具有手性单齿或双齿磷配体的铑配合物已成为不饱和酸不对称氢化的主要催化剂。但是,这些催化剂的效率高度依赖于底物,并且大多数报道的催化剂都需要高负载,高氢压,
更新日期:2017-04-04
中文翻译:

铱催化不饱和羧酸的不对称加氢
手性羧酸部分广泛用于药物,农用化学品,调味剂,香料和保健补品中。尽管它们可以通过过渡金属催化的不饱和羧酸的对映选择性氢化直接合成,但是由于现有的手性催化剂具有各种缺点,因此开发具有高活性和对映选择性的新型手性催化剂是一项重要的长期挑战。具有手性二膦配体的钌配合物和具有手性单齿或双齿磷配体的铑配合物已成为不饱和酸不对称氢化的主要催化剂。但是,这些催化剂的效率高度依赖于底物,并且大多数报道的催化剂都需要高负载,高氢压,











































 京公网安备 11010802027423号
京公网安备 11010802027423号