当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ring Construction by Palladium(0)-Catalyzed C(sp3)–H Activation
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-04-04 00:00:00 , DOI: 10.1021/acs.accounts.7b00099 Olivier Baudoin 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-04-04 00:00:00 , DOI: 10.1021/acs.accounts.7b00099 Olivier Baudoin 1
Affiliation
|
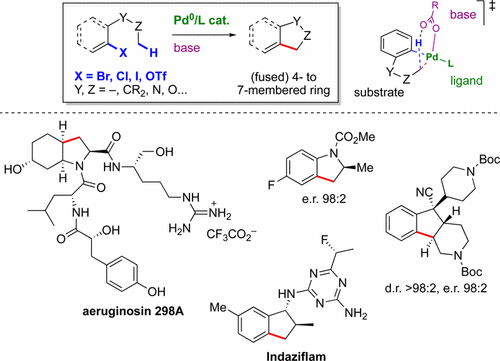
The catalytic activation and functionalization of unactivated C(sp3)–H bonds of alkyl groups has undergone intense development in recent years. In particular, a variety of directing groups as well as native functional groups have been employed in combination with palladium(II) catalysis in order to perform a variety of intermolecular, and to some extent intramolecular reactions. In parallel, inspired by precedents in C(sp2)–H arylation, our group and others have developed a different approach, which is the focus of this Account. This strategy relies on the use of oxidative addition of a carbon-leaving group bond to palladium(0) to induce intramolecular C(sp3)–H activation and the subsequent formation of a C(sp2)–C(sp3) or C(sp3)–C(sp3) bond. Since our first publication in 2003, the construction of olefins and, more interestingly, of an array of valuable monocyclic and polycyclic systems has been reported according to this principle. (Hetero)aryl bromides were initially employed as reactants, but the scope was later expanded to include (hetero)aryl chlorides and triflates, alkenyl bromides, carbamoyl chlorides and α-chloroamides. Mechanistic studies enabled a better understanding of the C–H activation step, which was proposed to occur through ambiphilic metal–ligand activation-6 (AMLA-6), also known as concerted metalation deprotonation (CMD), and a better rationalization of the observed selectivity patterns. Moreover, the wealth of accumulated experimental data indicate that the number of atoms separating the C–H bond from Pd and the type of C–H bond are the main factors controlling the site-selectivity of the C–H bond cleavage. Recent efforts have been devoted to the development of enantioselective reactions. To this purpose, two different strategies have been employed: a chiral ancillary ligand in combination with an achiral base, and a chiral base in combination with an achiral ligand, and allowed for the achievement of high enantioselectivities in the construction of both tri- and tetrasubstituted stereocenters. On the other hand, the current C–H activation-based ring-forming method was applied to the synthesis of pharmacologically active substances and agrochemicals, as well as complex natural products such as the aeruginosins, thereby demonstrating its great potential for step-economical organic synthesis.
中文翻译:

钯(0)催化的C(sp 3)–H活化环的构建
近年来,未活化的烷基C(sp 3)–H键的催化活化和功能化发展迅速。特别地,已经将各种指导基团以及天然官能团与钯(II)催化结合使用,以进行各种分子间反应,并且在某种程度上进行分子内反应。同时,受C(sp 2)–H芳构化的先例启发,我们小组和其他人开发了一种不同的方法,这是本帐户的重点。该策略依赖于对钯(0)的氧化成碳基团的氧化加成,以诱导分子内C(sp 3)–H活化并随后形成C(sp 2)–C(sp 3)或C(sp 3)–C(sp 3)债券。自从我们于2003年首次发表以来,据此原理报道了烯烃的构建,以及更有价值的是,有价值的单环和多环体系的阵列。最初使用(杂)芳基溴化物作为反应物,但后来范围扩大到包括(杂)芳基氯和三氟甲磺酸酯,烯基溴化物,氨基甲酰氯和α-氯酰胺。机理研究使人们可以更好地了解C–H活化步骤,该步骤建议通过歧义金属-配体活化6(AMLA-6)(也称为协同金属去质子化(CMD))进行,并且可以更好地合理观察到选择性模式。而且,大量积累的实验数据表明,将C–H键与Pd分开的原子数和C–H键的类型是控制C–H键裂解位点选择性的主要因素。最近的努力已经致力于对映选择性反应的发展。为了这个目的,已经采用了两种不同的策略:手性辅助配体与非手性碱结合,和手性碱与非手性配体结合,并允许在三取代和四取代的构建中实现高对映选择性。立体中心。另一方面,目前基于C–H活化的成环方法已应用于药理活性物质和农用化学品以及复杂的天然产物(如铜绿素)的合成。
更新日期:2017-04-04
中文翻译:

钯(0)催化的C(sp 3)–H活化环的构建
近年来,未活化的烷基C(sp 3)–H键的催化活化和功能化发展迅速。特别地,已经将各种指导基团以及天然官能团与钯(II)催化结合使用,以进行各种分子间反应,并且在某种程度上进行分子内反应。同时,受C(sp 2)–H芳构化的先例启发,我们小组和其他人开发了一种不同的方法,这是本帐户的重点。该策略依赖于对钯(0)的氧化成碳基团的氧化加成,以诱导分子内C(sp 3)–H活化并随后形成C(sp 2)–C(sp 3)或C(sp 3)–C(sp 3)债券。自从我们于2003年首次发表以来,据此原理报道了烯烃的构建,以及更有价值的是,有价值的单环和多环体系的阵列。最初使用(杂)芳基溴化物作为反应物,但后来范围扩大到包括(杂)芳基氯和三氟甲磺酸酯,烯基溴化物,氨基甲酰氯和α-氯酰胺。机理研究使人们可以更好地了解C–H活化步骤,该步骤建议通过歧义金属-配体活化6(AMLA-6)(也称为协同金属去质子化(CMD))进行,并且可以更好地合理观察到选择性模式。而且,大量积累的实验数据表明,将C–H键与Pd分开的原子数和C–H键的类型是控制C–H键裂解位点选择性的主要因素。最近的努力已经致力于对映选择性反应的发展。为了这个目的,已经采用了两种不同的策略:手性辅助配体与非手性碱结合,和手性碱与非手性配体结合,并允许在三取代和四取代的构建中实现高对映选择性。立体中心。另一方面,目前基于C–H活化的成环方法已应用于药理活性物质和农用化学品以及复杂的天然产物(如铜绿素)的合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号