当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Creating a Homeodomain with High Stability and DNA Binding Affinity by Sequence Averaging
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-28 , DOI: 10.1021/jacs.6b11323 Katherine W. Tripp 1 , Matt Sternke 1 , Ananya Majumdar 1 , Doug Barrick 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-28 , DOI: 10.1021/jacs.6b11323 Katherine W. Tripp 1 , Matt Sternke 1 , Ananya Majumdar 1 , Doug Barrick 1
Affiliation
|
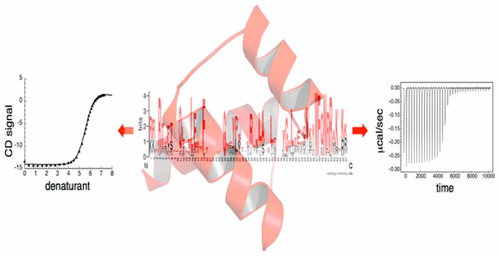
There is considerable interest in generating proteins with both high stability and high activity for biomedical and industrial purposes. One approach that has been used successfully to increase the stability of linear repeat proteins is consensus design. It is unclear the extent over which the consensus design approach can be used to produce folded and hyperstable proteins, and importantly, whether such stabilized proteins would retain function. Here we extend the consensus strategy to design a globular protein. We show that a consensus-designed homeodomain (HD) sequence adopts a cooperatively folded homeodomain structure. The unfolding free energy of the consensus-HD is 5 kcal·mol-1 higher than that of the naturally occurring engrailed-HD from Drosophila melanogaster. Remarkably, the consensus-HD binds the engrailed-HD cognate DNA in a similar mode as the engrailed-HD with approximately 100-fold higher affinity. 15N relaxation studies show a decrease in ps-ns backbone dynamics in the free state of consensus-HD, suggesting that increased affinity is not a result of increased plasticity. In addition to demonstrating the potential for consensus design of globular proteins with increased stability, these results demonstrate that greatly stabilized proteins can bind cognate substrates with increased affinities, showing that high stability is compatible with function.
中文翻译:

通过序列平均创建具有高稳定性和 DNA 结合亲和力的同源域
产生用于生物医学和工业目的的具有高稳定性和高活性的蛋白质引起了相当大的兴趣。已成功用于增加线性重复蛋白稳定性的一种方法是共有设计。目前尚不清楚共识设计方法可用于生产折叠和超稳定蛋白质的程度,重要的是,此类稳定蛋白质是否会保留功能。在这里,我们扩展了共识策略来设计球状蛋白质。我们展示了一致设计的同源域 (HD) 序列采用协同折叠的同源域结构。一致-HD 的展开自由能比黑腹果蝇天然存在的 engrailed-HD 高 5 kcal·mol-1。值得注意的是,共有-HD 以与 engrailed-HD 相似的模式结合 engrailed-HD 同源 DNA,亲和力高出约 100 倍。15N 松弛研究表明,在共识-HD 的自由状态下 ps-ns 骨架动力学下降,这表明亲和力增加并不是可塑性增加的结果。除了证明具有更高稳定性的球状蛋白质的共有设计的潜力之外,这些结果还表明,高度稳定的蛋白质可以以增加的亲和力结合同源底物,表明高稳定性与功能相容。
更新日期:2017-03-28
中文翻译:

通过序列平均创建具有高稳定性和 DNA 结合亲和力的同源域
产生用于生物医学和工业目的的具有高稳定性和高活性的蛋白质引起了相当大的兴趣。已成功用于增加线性重复蛋白稳定性的一种方法是共有设计。目前尚不清楚共识设计方法可用于生产折叠和超稳定蛋白质的程度,重要的是,此类稳定蛋白质是否会保留功能。在这里,我们扩展了共识策略来设计球状蛋白质。我们展示了一致设计的同源域 (HD) 序列采用协同折叠的同源域结构。一致-HD 的展开自由能比黑腹果蝇天然存在的 engrailed-HD 高 5 kcal·mol-1。值得注意的是,共有-HD 以与 engrailed-HD 相似的模式结合 engrailed-HD 同源 DNA,亲和力高出约 100 倍。15N 松弛研究表明,在共识-HD 的自由状态下 ps-ns 骨架动力学下降,这表明亲和力增加并不是可塑性增加的结果。除了证明具有更高稳定性的球状蛋白质的共有设计的潜力之外,这些结果还表明,高度稳定的蛋白质可以以增加的亲和力结合同源底物,表明高稳定性与功能相容。



























 京公网安备 11010802027423号
京公网安备 11010802027423号