当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unbinding Kinetics of a p38 MAP Kinase Type II Inhibitor from Metadynamics Simulations
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.6b12950 Rodrigo Casasnovas 1 , Vittorio Limongelli 2, 3 , Pratyush Tiwary 4 , Paolo Carloni 1 , Michele Parrinello 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.6b12950 Rodrigo Casasnovas 1 , Vittorio Limongelli 2, 3 , Pratyush Tiwary 4 , Paolo Carloni 1 , Michele Parrinello 5
Affiliation
|
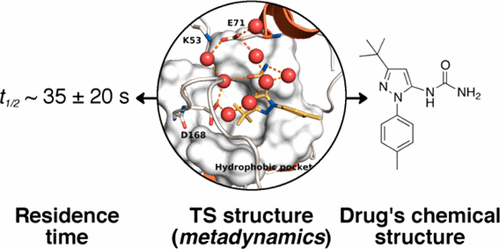
Understanding the structural and energetic requisites of ligand binding toward its molecular target is of paramount relevance in drug design. In recent years, atomistic free energy calculations have proven to be a valid tool to complement experiments in characterizing the thermodynamic and kinetic properties of protein/ligand interaction. Here, we investigate, through a recently developed metadynamics-based protocol, the unbinding mechanism of an inhibitor of the pharmacologically relevant target p38 MAP kinase. We provide a thorough description of the ligand unbinding pathway identifying the most stable binding mode and other thermodynamically relevant poses. From our simulations, we estimated the unbinding rate as koff = 0.020 ± 0.011 s-1. This is in good agreement with the experimental value (koff = 0.14 s-1). Next, we developed a Markov state model that allowed identifying the rate-limiting step of the ligand unbinding process. Our calculations further show that the solvation of the ligand and that of the active site play crucial roles in the unbinding process. This study paves the way to investigations on the unbinding dynamics of more complex p38 inhibitors and other pharmacologically relevant inhibitors in general, demonstrating that metadynamics can be a powerful tool in designing new drugs with engineered binding/unbinding kinetics.
中文翻译:

来自 Metadynamics 模拟的 p38 MAP 激酶 II 型抑制剂的解结合动力学
了解配体与其分子靶标结合的结构和能量要求在药物设计中至关重要。近年来,原子自由能计算已被证明是补充表征蛋白质/配体相互作用的热力学和动力学特性的实验的有效工具。在这里,我们通过最近开发的基于元动力学的协议研究了药理学相关靶标 p38 MAP 激酶抑制剂的解绑机制。我们提供了配体解结合途径的全面描述,确定了最稳定的结合模式和其他热力学相关姿势。根据我们的模拟,我们估计解绑定率为 koff = 0.020 ± 0.011 s-1。这与实验值非常吻合(koff = 0.14 s-1)。下一个,我们开发了一个马尔可夫状态模型,可以识别配体解绑过程的限速步骤。我们的计算进一步表明,配体的溶剂化和活性位点的溶剂化在解结合过程中起着至关重要的作用。这项研究为研究更复杂的 p38 抑制剂和其他药理学相关抑制剂的解结合动力学铺平了道路,表明元动力学可以成为设计具有工程结合/解结合动力学的新药的有力工具。
更新日期:2017-03-24
中文翻译:

来自 Metadynamics 模拟的 p38 MAP 激酶 II 型抑制剂的解结合动力学
了解配体与其分子靶标结合的结构和能量要求在药物设计中至关重要。近年来,原子自由能计算已被证明是补充表征蛋白质/配体相互作用的热力学和动力学特性的实验的有效工具。在这里,我们通过最近开发的基于元动力学的协议研究了药理学相关靶标 p38 MAP 激酶抑制剂的解绑机制。我们提供了配体解结合途径的全面描述,确定了最稳定的结合模式和其他热力学相关姿势。根据我们的模拟,我们估计解绑定率为 koff = 0.020 ± 0.011 s-1。这与实验值非常吻合(koff = 0.14 s-1)。下一个,我们开发了一个马尔可夫状态模型,可以识别配体解绑过程的限速步骤。我们的计算进一步表明,配体的溶剂化和活性位点的溶剂化在解结合过程中起着至关重要的作用。这项研究为研究更复杂的 p38 抑制剂和其他药理学相关抑制剂的解结合动力学铺平了道路,表明元动力学可以成为设计具有工程结合/解结合动力学的新药的有力工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号