当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies on Rhodium-Catalyzed Enantioselective Silylation of Aryl C–H Bonds
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.7b00737 Taegyo Lee 1 , John F. Hartwig 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-03-24 , DOI: 10.1021/jacs.7b00737 Taegyo Lee 1 , John F. Hartwig 1
Affiliation
|
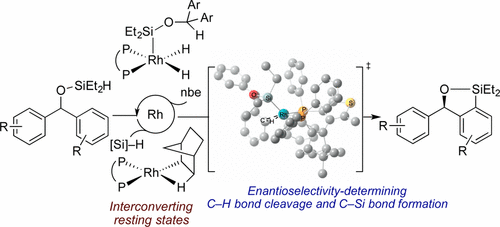
Several classes of enantioselective silylations of C-H bonds have been reported recently, but little mechanistic data on these processes are available. We report mechanistic studies on the rhodium-catalyzed, enantioselective silylation of aryl C-H bonds. A rhodium silyl dihydride and a rhodium norbornyl complex were prepared and determined to be interconverting catalyst resting states. Kinetic isotope effects indicated that the C-H bond cleavage step is not rate-determining, but the C-H bond cleavage and C-Si bond-forming steps together influence the enantioselectivity. DFT calculations indicate that the enantioselectivity originates from unfavorable steric interactions between the substrate and the ligand in the transition state leading to the formation of the minor enantiomer.
中文翻译:

铑催化芳基C-H键对映选择性硅烷化的机理研究
最近报道了几类 CH 键的对映选择性硅烷化,但关于这些过程的机械数据很少。我们报告了铑催化的芳基 CH 键对映选择性硅烷化的机理研究。制备铑甲硅烷基二氢化物和铑降冰片基络合物并确定为互变催化剂静止状态。动力学同位素效应表明 CH 键裂解步骤不是决定速率的,但 CH 键裂解和 C-Si 键形成步骤共同影响对映选择性。DFT 计算表明,对映选择性源于底物和过渡态配体之间不利的空间相互作用,导致形成少量对映异构体。
更新日期:2017-03-24
中文翻译:

铑催化芳基C-H键对映选择性硅烷化的机理研究
最近报道了几类 CH 键的对映选择性硅烷化,但关于这些过程的机械数据很少。我们报告了铑催化的芳基 CH 键对映选择性硅烷化的机理研究。制备铑甲硅烷基二氢化物和铑降冰片基络合物并确定为互变催化剂静止状态。动力学同位素效应表明 CH 键裂解步骤不是决定速率的,但 CH 键裂解和 C-Si 键形成步骤共同影响对映选择性。DFT 计算表明,对映选择性源于底物和过渡态配体之间不利的空间相互作用,导致形成少量对映异构体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号