当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution and Substrate Specificity of the Radical S-Adenosyl-methionine Thiazole C-Methyltransferase in Thiomuracin Biosynthesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-21 , DOI: 10.1021/jacs.7b00693 Nilkamal Mahanta 1, 2 , Zhengan Zhang 1 , Graham A Hudson 1 , Wilfred A van der Donk 1, 2, 3 , Douglas A Mitchell 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-21 , DOI: 10.1021/jacs.7b00693 Nilkamal Mahanta 1, 2 , Zhengan Zhang 1 , Graham A Hudson 1 , Wilfred A van der Donk 1, 2, 3 , Douglas A Mitchell 1, 2
Affiliation
|
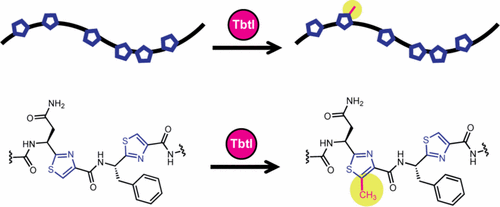
Thiomuracin is a thiopeptide antibiotic with potent activity toward Gram-positive drug-resistant bacteria. Thiomuracin is biosynthesized from a precursor peptide, TbtA, by a complex array of posttranslational modifications. One of several intriguing transformations is the C-methylation of thiazole, occurring at an unactivated sp2 carbon. Herein, we report the in vitro reconstitution of TbtI, the responsible radical S-adenosyl-methionine (rSAM) C-methyltransferase, which catalyzes the formation of 5-methylthiazole at a single site. Our studies demonstrate that a linear hexazole-bearing intermediate of TbtA is a substrate for TbtI whereas macrocyclized thiomuracin GZ is not. In determining the minimal substrate for TbtI, we found that the enzyme is functional when most of the leader peptide has been removed. The in vitro reconstitution of TbtI, a class C rSAM methyltransferase, further adds to the chemical versatility of rSAM enzymes, and informs on the complexity of thiomuracin biosynthesis.
中文翻译:

硫霉素生物合成中自由基 S-腺苷甲硫氨酸噻唑 C-甲基转移酶的重建和底物特异性
Thiomuracin 是一种硫肽抗生素,对革兰氏阳性耐药菌具有有效活性。硫霉素是由前体肽 TbtA 通过一系列复杂的翻译后修饰生物合成的。几个有趣的转变之一是噻唑的 C-甲基化,发生在未活化的 sp2 碳上。在此,我们报告了 TbtI 的体外重建,TbtI 是负责的自由基 S-腺苷甲硫氨酸 (rSAM) C-甲基转移酶,可在单个位点催化 5-甲基噻唑的形成。我们的研究表明,TbtA 的线性六唑中间体是 TbtI 的底物,而大环化硫村霉素 GZ 则不是。在确定 TbtI 的最小底物时,我们发现当大部分前导肽被去除时,该酶才发挥作用。TbtI(一种 C 类 rSAM 甲基转移酶)的体外重构进一步增加了 rSAM 酶的化学多功能性,并揭示了硫胞菌素生物合成的复杂性。
更新日期:2017-03-21
中文翻译:

硫霉素生物合成中自由基 S-腺苷甲硫氨酸噻唑 C-甲基转移酶的重建和底物特异性
Thiomuracin 是一种硫肽抗生素,对革兰氏阳性耐药菌具有有效活性。硫霉素是由前体肽 TbtA 通过一系列复杂的翻译后修饰生物合成的。几个有趣的转变之一是噻唑的 C-甲基化,发生在未活化的 sp2 碳上。在此,我们报告了 TbtI 的体外重建,TbtI 是负责的自由基 S-腺苷甲硫氨酸 (rSAM) C-甲基转移酶,可在单个位点催化 5-甲基噻唑的形成。我们的研究表明,TbtA 的线性六唑中间体是 TbtI 的底物,而大环化硫村霉素 GZ 则不是。在确定 TbtI 的最小底物时,我们发现当大部分前导肽被去除时,该酶才发挥作用。TbtI(一种 C 类 rSAM 甲基转移酶)的体外重构进一步增加了 rSAM 酶的化学多功能性,并揭示了硫胞菌素生物合成的复杂性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号