当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-21 , DOI: 10.1021/jacs.7b01128 Feng Gao 1 , Donghai Mei 1 , Yilin Wang 1 , János Szanyi 1 , Charles H. F. Peden 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-21 , DOI: 10.1021/jacs.7b01128 Feng Gao 1 , Donghai Mei 1 , Yilin Wang 1 , János Szanyi 1 , Charles H. F. Peden 1
Affiliation
|
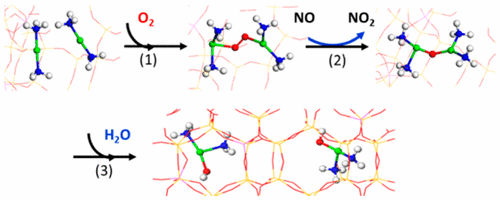
Active centers in Cu/SSZ-13 selective catalytic reduction (SCR) catalysts have been recently identified as isolated Cu2+ and [CuII(OH)]+ ions. A redox reaction mechanism has also been established, where Cu ions cycle between CuI and CuII oxidation states during SCR reaction. While the mechanism for the reduction half-cycle (CuII → CuI) is reasonably well-understood, that for the oxidation half-cycle (CuI → CuII) remains an unsettled debate. Herein we report detailed reaction kinetics on low-temperature standard NH3-SCR, supplemented by DFT calculations, as strong evidence that the low-temperature oxidation half-cycle occurs with the participation of two isolated CuI ions via formation of a transient [CuI(NH3)2]+-O2-[CuI(NH3)2]+ intermediate. The feasibility of this reaction mechanism is confirmed from DFT calculations, and the simulated energy barrier and rate constants are consistent with experimental findings. Significantly, the low-temperature standard SCR mechanism proposed here provides full consistency with low-temperature SCR kinetics.
中文翻译:

Cu/SSZ-13 上的选择性催化还原:连接均相和多相催化
Cu/SSZ-13 选择性催化还原 (SCR) 催化剂中的活性中心最近被确定为孤立的 Cu2+ 和 [CuII(OH)]+ 离子。还建立了氧化还原反应机制,其中 Cu 离子在 SCR 反应期间在 CuI 和 CuII 氧化态之间循环。虽然还原半循环 (CuII → CuI) 的机制已被合理理解,但氧化半循环 (CuI → CuII) 的机制仍然是一个悬而未决的争论。在此,我们报告了低温标准 NH3-SCR 的详细反应动力学,并辅以 DFT 计算,作为强有力的证据表明,低温氧化半循环是在两个孤立的 CuI 离子通过形成瞬态 [CuI(NH3 )2]+-O2-[CuI(NH3)2]+ 中间体。DFT计算证实了该反应机理的可行性,并且模拟的能垒和速率常数与实验结果一致。值得注意的是,这里提出的低温标准 SCR 机制与低温 SCR 动力学完全一致。
更新日期:2017-03-21
中文翻译:

Cu/SSZ-13 上的选择性催化还原:连接均相和多相催化
Cu/SSZ-13 选择性催化还原 (SCR) 催化剂中的活性中心最近被确定为孤立的 Cu2+ 和 [CuII(OH)]+ 离子。还建立了氧化还原反应机制,其中 Cu 离子在 SCR 反应期间在 CuI 和 CuII 氧化态之间循环。虽然还原半循环 (CuII → CuI) 的机制已被合理理解,但氧化半循环 (CuI → CuII) 的机制仍然是一个悬而未决的争论。在此,我们报告了低温标准 NH3-SCR 的详细反应动力学,并辅以 DFT 计算,作为强有力的证据表明,低温氧化半循环是在两个孤立的 CuI 离子通过形成瞬态 [CuI(NH3 )2]+-O2-[CuI(NH3)2]+ 中间体。DFT计算证实了该反应机理的可行性,并且模拟的能垒和速率常数与实验结果一致。值得注意的是,这里提出的低温标准 SCR 机制与低温 SCR 动力学完全一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号