当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Are Ionic Liquids Chemically Stable?
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-02-27 00:00:00 , DOI: 10.1021/acs.chemrev.6b00594 Binshen Wang 1 , Li Qin 1 , Tiancheng Mu 2 , Zhimin Xue 3 , Guohua Gao 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-02-27 00:00:00 , DOI: 10.1021/acs.chemrev.6b00594 Binshen Wang 1 , Li Qin 1 , Tiancheng Mu 2 , Zhimin Xue 3 , Guohua Gao 1
Affiliation
|
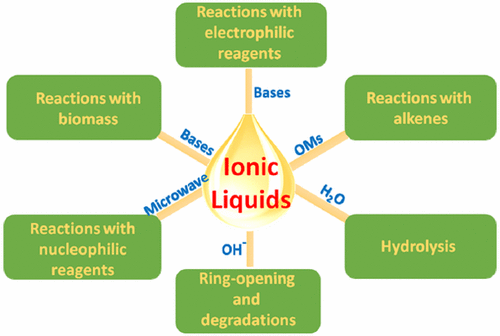
Ionic liquids have attracted a great deal of interest in recent years, illustrated by their applications in a variety of areas involved with chemistry, physics, biology, and engineering. Usually, the stabilities of ionic liquids are highlighted as one of their outstanding advantages. However, are ionic liquids really stable in all cases? This review covers the chemical stabilities of ionic liquids. It focuses on the reactivity of the most popular imidazolium ionic liquids at structural positions, including C2 position, N1 and N3 positions, and C4 and C5 positions, and decomposition on the imidazolium ring. Additionally, we discuss decomposition of quaternary ammonium and phosphonium ionic liquids and hydrolysis and nucleophilic reactions of anions of ionic liquids. The review aims to arouse caution on potential decomposition of ionic liquids and provides a guide for better utilization of ionic liquids.
中文翻译:

离子液体化学稳定吗?
近年来,离子液体引起了人们的极大兴趣,其在化学,物理,生物学和工程学等各个领域的应用都证明了这一点。通常,离子液体的稳定性被强调为其突出优点之一。但是,离子液体在所有情况下真的稳定吗?这篇综述涵盖了离子液体的化学稳定性。它着眼于最流行的咪唑鎓离子液体在结构位置(包括C2位置,N1和N3位置以及C4和C5位置)的反应性,以及在咪唑环上的分解。此外,我们讨论了季铵和phospho离子液体的分解以及离子液体阴离子的水解和亲核反应。
更新日期:2017-02-27
中文翻译:

离子液体化学稳定吗?
近年来,离子液体引起了人们的极大兴趣,其在化学,物理,生物学和工程学等各个领域的应用都证明了这一点。通常,离子液体的稳定性被强调为其突出优点之一。但是,离子液体在所有情况下真的稳定吗?这篇综述涵盖了离子液体的化学稳定性。它着眼于最流行的咪唑鎓离子液体在结构位置(包括C2位置,N1和N3位置以及C4和C5位置)的反应性,以及在咪唑环上的分解。此外,我们讨论了季铵和phospho离子液体的分解以及离子液体阴离子的水解和亲核反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号