当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism.
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-02-24 , DOI: 10.1021/acs.chemrev.6b00737 Laurent Cappadocia 1 , Christopher D Lima 1, 2
Chemical Reviews ( IF 62.1 ) Pub Date : 2017-02-24 , DOI: 10.1021/acs.chemrev.6b00737 Laurent Cappadocia 1 , Christopher D Lima 1, 2
Affiliation
|
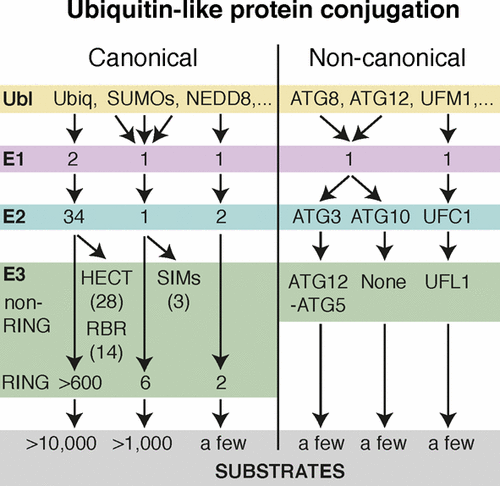
Ubiquitin-like proteins (Ubl's) are conjugated to target proteins or lipids to regulate their activity, stability, subcellular localization, or macromolecular interactions. Similar to ubiquitin, conjugation is achieved through a cascade of activities that are catalyzed by E1 activating enzymes, E2 conjugating enzymes, and E3 ligases. In this review, we will summarize structural and mechanistic details of enzymes and protein cofactors that participate in Ubl conjugation cascades. Precisely, we will focus on conjugation machinery in the SUMO, NEDD8, ATG8, ATG12, URM1, UFM1, FAT10, and ISG15 pathways while referring to the ubiquitin pathway to highlight common or contrasting themes. We will also review various strategies used to trap intermediates during Ubl activation and conjugation.
中文翻译:

泛素样蛋白结合:结构,化学和机理。
泛素样蛋白(Ubl's)与目标蛋白或脂质缀合,以调节其活性,稳定性,亚细胞定位或大分子相互作用。与泛素类似,通过E1活化酶,E2缀合酶和E3连接酶催化的一系列活性来实现缀合。在这篇综述中,我们将总结参与Ubl偶联级联反应的酶和蛋白质辅因子的结构和机理细节。确切地说,我们将重点介绍SUMO,NEDD8,ATG8,ATG12,URM1,UFM1,FAT10和ISG15途径中的缀合机制,同时提及泛素途径以强调常见或相反的主题。我们还将回顾在Ubl激活和结合过程中用于捕获中间体的各种策略。
更新日期:2017-02-24
中文翻译:

泛素样蛋白结合:结构,化学和机理。
泛素样蛋白(Ubl's)与目标蛋白或脂质缀合,以调节其活性,稳定性,亚细胞定位或大分子相互作用。与泛素类似,通过E1活化酶,E2缀合酶和E3连接酶催化的一系列活性来实现缀合。在这篇综述中,我们将总结参与Ubl偶联级联反应的酶和蛋白质辅因子的结构和机理细节。确切地说,我们将重点介绍SUMO,NEDD8,ATG8,ATG12,URM1,UFM1,FAT10和ISG15途径中的缀合机制,同时提及泛素途径以强调常见或相反的主题。我们还将回顾在Ubl激活和结合过程中用于捕获中间体的各种策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号