2025
283. Yi-WenZhang, Zihao Ye, Dejun Hu, Shutao Qi, Zuobang Sun, Junfeng Yang, Yan Ma,Wayne Zhang,* Junliang Zhang,* and Zhiming Li*
QC-AugmentedNN/GNNforFew-ShotPredictionofAmorphous PolymerPropertiesViaMulti-ScaleMicrostructures
ACS Appl. Polym. Mater. 2025, XXXX, XXX, XXX-XXX. https://doi.org/10.1021/acsapm.5c01557

282. Ben Huang, Cao Li, Yuanjing Xiao*, Junliang Zhang*, Lu Liu*
Modular Synthesis of Chiral Trisubstituted 1,2-Allenyl Ketones Enabled by Organophosphine Dual-Reagent Catalysis
Chin. J. Chem. 2026, 44, 33—39

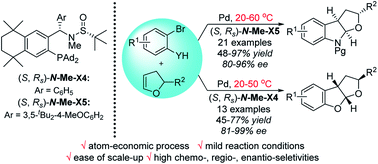
281. Yin Yuan , Liangkai Pan , Yidan Han, Xinyu Tian, Wenshao Ye , Junliang Zhang* , Junfeng Yang*
CCS Chemistry. DOI: 10.31635/ccschem.025.202505937

280. Zhaoqiang Chen,∥Huanan Wang,∥Ping Du, Jiaao Zhao, Xue Zhang,* Hui Qian,*Junliang Zhang,* and Shengming Ma*
DACH-ZYC-Phos/Pd-CatalyzedEnantioselectiveAllenylationof SecondaryPhosphineOxidesviaLigandRelay
J.Am.Chem.Soc. 2025, 147, 24958−24968

PC-Phos
279. Chun Ma, Xinyue Wang, Tibor Soós, Junliang Zhang*, Junfeng Yang*
Ligand-controlled regiodivergent and enantioselective hydrophosphorylation of styrenes by palladium

278. Huamin Wang*, Hong-Ding Xie, Jun-Long Ding, Yanjun Xie, Ying-Wu Lin*, Junliang Zhang*
Phosphine-Catalyzed Asymmetric Dearomative Annulation of 4‑Nitroisoxazoles with Allenoates: Construction of Isoxazoline-Fused Bicyclo[3.3.0]octenes
Org. Lett. 2025, 27, 6812–6816

277. Yangwei Xia, Bing Xu, Zhanming Zhang* and Junliang Zhang*
Pd/Ag dual-catalyzed asymmetric synthesis of sulfur-stereogenic sulfoximines via enantioselective intramolecular C−H arylation
Green Synthesis and Catalysis. DOI: org/10.1016/j.gresc.2025.06.004

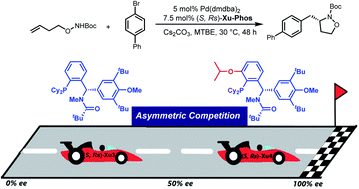
276. Kexin Dong, Chao Fang, Zhongyu Li, Bing Xu, Zhanming Zhang* and Junliang Zhang*
Palladium/Xu-Phos-Catalyzed Enantioselective Cascade Heck/Intermolecular Direct Heteroarylation Reaction
Chin. J. Chem. 2025, 43, 2199—2205

- 275. Yin Yuan, Xinyu Tian, Hongxia Zheng, Yuze Li, Junliang Zhang,* and Junfeng Yang*
Catalytic Enantioselective Reductive Arylation and Alkenylationof Sulfinylamines to Access Sulfinamides Enabled by CobaltCatalysis
Angew. Chem. Int. Ed. 2025, e202506243. DOI:org/10.1002/anie.202506243
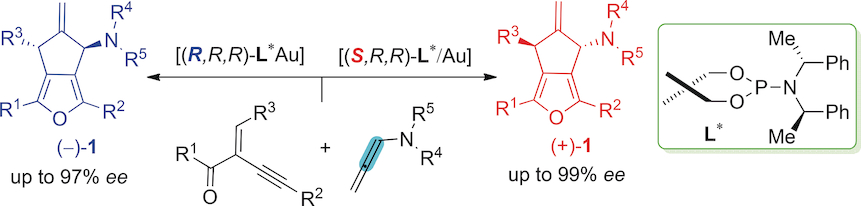
274. Yang-Wei Xia, Bing Xu, Zhan-Ming Zhang*, Junliang Zhang*
Construction of Novel Chiral NPN Ligands and the Application in Gold(I)-Catalyzed Asymmetric Cycloaddition Reactions
Chem. Eur. J. 2025, 0, e202500465

- 273. Yidong Wang*,
- Hang Zhou,
- Yan Sun,
- Shenglong Wang,
- Ruiqiang Lu,
- Shiwan Sun,
- Ying Han,
- Liuzhou Gao*,
- Junliang Zhang*.
Org. Lett. 2025, XXXX, XXX, XXX-XXX. DOI:org/10.1021/acs.orglett.5c00911

272.Chenghao Zhu†, Zihao Ye†, Haining Wang, Da Ma, Zhiming Li*, Chao-Jun Li *, Junliang Zhang*
Chiral ruthenium complex/Ph2P(2- furyl)–catalyzedasymmetric nucleophilic addition of aryl aldehydehydrazones to simple ketones
Sci. Adv. 2025, 11, eadv0095.

271.Xinyu Zhu, Junliang Wu, *Junliang Zhang* and Junfeng Yang*
Photoredox-catalyzed deoxygenative radical transformation of alcohols to sulfinamides
RSC Adv., 2025,15, 4532-4535.

270. Li-Zhi Zhang, Pei-Chao Zhang, QianWang, Min Zhou*, and Junliang Zhang*
Enantioselective Heck/Tsuji−Trost reaction of flexible vinylic halides with 1,3-dienes.
Nat Commun. 2025, 16, 930.

- 269. Wenshao Ye,
- Kangning Cao,
- Zihao Ye,
- Shutao Qi,
- Junfeng Yang*, and
- Junliang Zhang*
-
- Enantioselective Palladium-Catalyzed Cascade Narasaka−HeckCyclization/Suzuki Coupling Reaction.
- ACS Catal. 2025, 15, XXX, 2516–2521, DOI:org/10.1021/acscatal.4c06658

- Enantioselective Palladium-Catalyzed Cascade Narasaka−HeckCyclization/Suzuki Coupling Reaction.
2024
268.Guan-Ming Chen, Zi-Hao Ye, Zhi-Ming Li,* and Jun-Liang Zhang*
Universal descriptors of quasi transition states for small-data-driven asymmetric catalysis prediction in machine learning model.
Cell Reports Physical Science 2024, 5, 102043. DOI: 10.1016/j.xcrp.2024.102043

267.Zhou Luo,‡ Tianxiang Fan,‡ Jingyan Luo, Yuanyuan Liu * and Junliang Zhang*
Ir/XuPhos-catalyzed direct asymmetric reductive amination of ketones with secondary amines.
Org. Chem. Front.,2024,11,6735–6741.

266. Zhi-Kun Zhang, Yin Yuan, Huiling Peng, Yidan Han, Junliang Zhang*, Junfeng Yang*
Synthesis of Sulfinamidines via Iron-Catalyzed Nitrene Transfer Reaction with Sulfenamides.
J. Org. Chem. 2024, XXXX, XXX-XXX, DOI:.org/10.1021/acs.joc.4c02286.

265.Bing Xu, Danting Ji, Zhan-Ming Zhang, * Junliang Zhang*
Remote C─H Bond Activation via Enantioselective Carbopalladation and 1,4-Pd Migration Cascade Process.
-
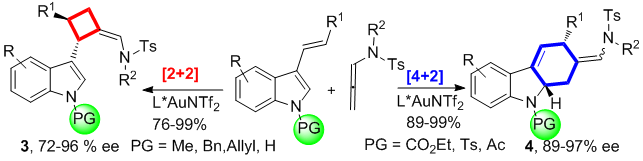
- 264. Kangning Cao, Jie Han, Wenshao Ye, Dejun Hu, Zihao Ye, Junfeng Yang,* Junliang Zhang,* and Fener Chen*
Enantioselective Aminosilylation of Alkenes byPalladium/Ming-Phos-Catalyzed TandemNarasaka–Heck/Silylation Reaction.
- Ming-Phos
-
-

- 263.
- Jinrong Wang,
- Bing Xu,
- Yibo Wang,
- Guangzhen Xia,
- Zhan-Ming Zhang
- *
- , and
- Junliang Zhang*
-
Pd-Catalyzed Enantioselective Three-Component Carboaminationof 1,3-Cyclohexadiene.
- 261. Yixiang Shi
- ,
- Yin Yuan
- ,
- Jianhui Li
- ,
- Junfeng Yang*
- , and
- Junliang Zhang*
Catalytic Asymmetric Synthesis of Sulfinamides via Cu-Catalyzed Asymmetric Addition of Aryl Boroxines to Sulfinylamines.
J. Am. Chem. Soc. 2024, XXXX, XXX, XXX-XXX, DOI:10.1021/jacs.4c03473.

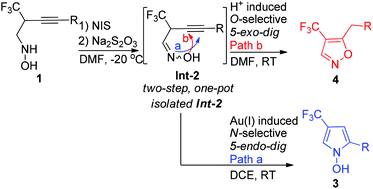
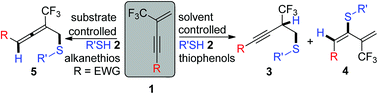
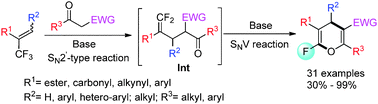
260. Qiaojing Meng, Yinggao Meng, Qinglin Liu, Bing Yu, Zhong-Jun Li, Er-Qing Li*, Junliang Zhang*

J. Am. Chem. Soc. 2024, XXXX, XXX-XXX, DOI: 10.1021/jacs.4c01851.

258. Lujia Zhou, Longling Ma, Bing Xu, Zhan-Ming Zhang*, Junliang Zhang*
Palladium/Xu-Phos Catalyzed Enantioselective Intramolecular Heck Reaction of Unactivated Alkenes.
Chin. J. Chem. 2024, 42, 2466—2470, DOI: 10.1002/cjoc.202400391.

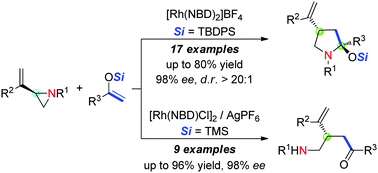
Ligand-Controlled, Nickel-Catalyzed Stereodivergent Construction of 1,3-Nonadjacent Stereocenters.
J. Am. Chem. Soc. 2024, 146, 22, 15453–15463.

256. Mengyuan Liu, Kexin Dong, Bing Xu, Zhan-Ming Zhang*, Zhong Wei *, and Junliang Zhang *
Nickel(0)-catalyzed ring-opening reaction of silacyclobutanes with 1,3-dienes to access allylsilane.
Org. Chem. Front., 2024, DOI: 10.1039/D4QO00357H.
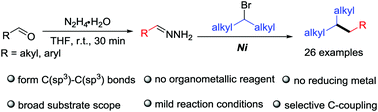
Copper-Catalyzed Sulfur Alkylation of Sulfenamides with N-Sulfonylhydrazones
Org. Lett. 2024, 26, 18, 3906–3910.

254. Kangning Cao#, Shutao Qi#, Jianhui Li , Wenshao Ye, Junfeng Yang*, Junliang Zhang*, Fener Chen*
Palladium-catalyzed tandem aza-Heck reaction of alkene-tethered oxime esters with cyclopropanols.
Green Synthesis and Catalysis, 2024,DOI: 10.1016/j.gresc.2024.04.002.

253. Shuai Zhu#, Mingjie Chen#,Haichao Shen, Hanming Ding*, Wenbo Li*, Junliang Zhang*
Chinese Chemical Letters, 2024, 109879. DOI: 10.1016/j.cclet.2024.109879.
Xu-Phos

252. Zhao Yongzhe, Kong Xiangfei*, Zhang Junliang*, Yang Junfeng*
Research Progress in the Application of Supported Catalysts Containing Phosphine.
Chin. J. Org. Chem. 2024, 44, xxxx~xxxx, DOI: 10.6023/cjoc202403016.

251. Chao Fang, Quan-Pu Wang, Bing Xu, Zhan-Ming Zhang* and Junliang Zhang*
Palladium/Xu-Phos-catalyzed enantioselective cascade Heck/intermolecular C(sp2)–H alkylation reaction
Chem. Sci., 2024,15, 5573-5580
Xu-Phos
- 250. Kangning Cao#
- ,
- Jie Han#
- ,
- Haichao Shen
- ,
- Junfeng Yang*
- ,
- Junliang Zhang*
- , and
- Fener Chen*
Pd-Catalyzed Asymmetric Aza-Heck Cyclization/Sonogashira Reaction of Alkene-Tethered Oxime Esters and Alkynes.
ACS Catal. 2024, 14, 5305–5313.
Ming-Phos

249. Genwei Zhang, Bin Yang, Junfeng Yang*, and Junliang Zhang*
Pd-Catalyzed Asymmetric Larock Indole Synthesis to Access Axially Chiral N-Arylindoles.
J. Am. Chem. Soc. 2024, 146, 5493–5501.

J. Am. Chem. Soc. 2024, 146, 4320–4326

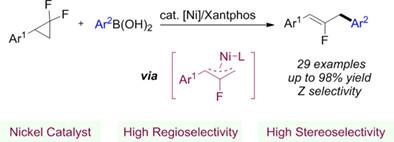
247. Shutao Qi, Yunkai Hua, Liangkai Pan, Junfeng Yang*, Junliang Zhang*
Chin. J. Chem. 2024, 42, 823-828
246. Wenbo Li and Junliang Zhang*
Sadphos as Adaptive Ligands in Asymmetric Palladium Catalysis.
Acc. Chem. Res. 2024, 57, 489–513.

2023年
Nature Communications | (2023) 14:7611, DOI: 10.1038/s41467-023-43202-5
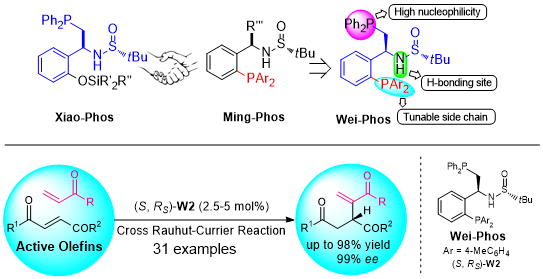
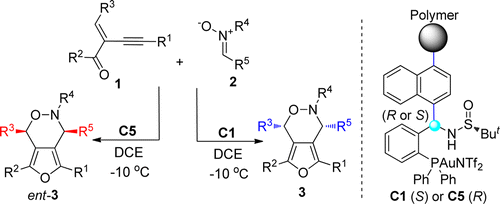
244. Shutao Qi, Wenshao Ye, Yunkai Hua, Liangkai Pan, Junfeng Yang*, and Junliang Zhang*
Nat. Synth. 2023, DOI: 10.1038/s44160-023-00448-7.
Xiang-Phos

243. Yue Sun, Kaida Zhou, Chun Ma, Zhiming Li*, and Junliang Zhang*
Green Synth. Catal. 2023, DOI: 10.1016/j.gresc.2023.09.001.
Ming-Phos

242. Hui Zhang, Bing Xu, Liejin Zhou , Zhan-Ming Zhang*, and Junliang Zhang*
Green Synth. Catal. 2023, DOI: 10.1016/j.gresc.2023.04.002.
Xu-Phos

241. Li-Ming Zhang, Wenjun Luo, Jiangzhen Fu, Yu Liu*, and Junliang Zhang*
ACS Catal. 2023, 13, XXX, 8830–8837. DOI: 10.1021/acscatal.3c01775
Sci. Adv. 2023, 9, eadg1002. DOI: 10.1126/sciadv.adg1002
(†These authors contributed equally to this work)
Xu-Phos

Angew. Chem.Int. Ed. 2023, 62, e202300309
Xu-Phos

238. Shiquan Gao, Chen Wang, Junfeng Yang* & Junliang Zhang*
Cobalt-catalyzed enantioselective intramolecular reductive cyclization via electrochemistry.

237. Youshao Tu, Bing Xu, Qian Wang, Honglin Dong, Zhan-Ming Zhang*, Junliang Zhang*
Palladium/TY-Phos-Catalyzed Asymmetric Heck/Tsuji–Trost Reaction of o-Bromophenols with 1,3-Dienes
J. Am. Chem. Soc. 2023, DOI: 10.1021/jacs.2c12752
TY-Phos

236. Ronghua Zhang, Shan Xu, Zhou Luo, Yuanyuan Liu,* and Junliang Zhang*
Angew. Chem. Int. Ed. 2023, e202213600
Rong-Phos

235. Chun Ma, Yue Sun, Junfeng Yang*, Hao Guo*, and Junliang Zhang*
Catalytic Asymmetric Synthesis of Tröger’s Base Analogues with Nitrogen Stereocenter
ACS Cent. Sci. 2023, DOI: 10.1021/acscentsci.2c01121
GF-Phos

234. Yin Yuan, Junfeng Yang* and Junliang Zhang*
Chem. Sci., 2023, DOI: 10.1039/D2SC05428K

2022年
Angew. Chem. Int. Ed. 2022, e202215407
(‡ These authors contributed equally to this work)
PC-Phos

232. Wenge Zhang‡, Pei-Chao Zhang‡, Yin-Lin Li, Hai-Hong Wu, and Junliang Zhang*
PC-Phos Enabled Catalytic Palladium-heteroallyl Asymmetric Cycloaddition
J. Am. Chem. Soc. 2022, 144, 19627–19634.
(‡ These authors contributed equally to this work)

231. Chun Ma, Yue Sun, Siyuan Liu, Zhi-Ming Li, Junfeng Yang*, Hao Guo*, Junliang Zhang.*
Chem Catalysis, 2022, 2, 3196-3206.
Xu-Phos



229. Yaochen Zhang, Zhangjin Pan, Hao Guo,* Junfeng Yang,* Junliang Zhang*
10 Gram-scale synthesis of TY-Phos ligand and its application in carbene insertion of Si-H bonds
Tetrahedron Lett., 2022, 107, 154120
TY-Phos

228. Honghao Bao, Yixiang Shi, Junliang Zhang,* Junfeng Yang* and Junliang Wu*.
Stereoselective synthesis of trisubstituted epoxides via cobalt catalysis.
Org. Chem. Front., 2022, 9, 4932-4936.
227. Yue Sun, Chun Ma, Zhiming Li,* Junliang Zhang*
Chem. Sci., 2022, 13, 11150-11155.
GF-Phos

226. Huanan Wang, Hui Qian*, Junliang Zhang*, and Shengming Ma*
Catalytic Asymmetric Axially Chiral Allenyl C–P Bond Formation.
J. Am. Chem. Soc. 2022, 144, 12619–12626.

225. Bin Yang, Kangning Cao, Guofeng Zhao, Junfeng Yang*, and Junliang Zhang*
J. Am. Chem. Soc. 2022, 144, 15468–15474.
Ming-Phos

224. Wenjun Luo, Li-Ming Zhang, Zhan-Ming Zhang, Junliang Zhang*.
Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202204443
W-Phos

223. Sanliang Li‡, Qiaoyu Chen‡, Junfeng Yang*, Junliang Zhang*.
Palladium-Catalyzed Enantioselective γ-Arylation of β,γ-Unsaturated Butenolides.
Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202202046.
(‡ These authors contributed equally to this work)

222. Bing Xu, Danting Ji, Lizuo Wu, Lujia Zhou, Yu Liu, Zhan-Ming Zhang*, Junliang Zhang*.
Palladium/Xu-Phos-catalyzed enantioselective cascade Heck/remote C(sp2) –H alkylation reaction.
Xu-Phos

Enantioselective Dearomative Mizoroki–Heck Reaction of Naphthalenes.
ACS Catal. 2022, 12, 655–661. (‡ These authors contributed equally to this work)
Xu-Phos

220. Bing Xu, Zhan-Ming Zhang, Jie Han, Guangxin Gu*, Junliang Zhang*.
Chin. J. Chem. 2022, 40, 1407-412. (封面文章)&(荣获“新和成《中国化学》创新奖", NHU-CJC Innovation Award)
Ming-Phos


219. Xiaoxiao Xie, Sanliang Li, Qiaoyu Chen, Hao Guo, * Junfeng Yang,* Junliang Zhang*.
Synthesis and application of novel P-chiral monophosphorus ligands.
Org. Chem. Front., 2022, 9, 1589-1592.
Xiao-Phos
2021年
218. Kangning Cao, Zhan-Ming Zhang, Junliang Zhang*, and Fener Chen*.
Palladium-Catalyzed Asymmetric Cross-Coupling Reactions of Cyclobutanols and Unactivated Olefins.
Org. Lett. 2021, 23, 9520–9525.
Xu-Phos

217. Sanliang Li‡,Qiaoyu Chen‡,Zhan-Ming Zhang,JunliangZhang*.
Green Synth. Catal. 2021, 2, 374. (‡ These authors contributed equally to this work)
Ming-Phos

216. Sanliang Li‡, Qiaoyu Chen‡, Xiaoxiao Xie, Junfeng Yang*, and Junliang Zhang*
Pd-Catalyzed Enantioselective Dearomative Allylic Annulation to Access PPAPs Analogues.
Org. Lett. 2021, 23, 7824. (‡ These authors contributed equally to this work)

215. Qiang Dai, Lu Liu,* Junliang Zhang*.
Palladium/Xiao-Phos-Catalyzed Kinetic Resolution of sec-Phosphine Oxides by P-Benzylation.
Angew. Chem. Int. Ed. 2021, 60, 27247.
(Dedicated to 70th anniversary of East China Normal University)
Xiao-Phos

214. Guofeng Zhao, Yi Wu, Hai-Hong Wu, Junfeng Yang*, and Junliang Zhang*.
J. Am. Chem. Soc. 2021, 143, 17983–17988.
GF-Phos

213. Yin-Lin Li,‡ Pei-Chao Zhang,‡ Hai-Hong Wu and Junliang Zhang.*
J. Am. Chem. Soc. 2021, 143, 13010–13015. (‡ These authors contributed equally to this work)
Xu-Phos & PC-Phos

212. Pei-Chao Zhang, Yin-Lin Li, Jiafeng He, Hai-Hong Wu,* Zhiming Li* & Junliang Zhang*.
TY-Phos

211. Zhangjin Pan, Wenbo Li, Shuai Zhu, Feng Liu, Hai-Hong Wu, Junliang Zhang*.
Palladium/TY-Phos-Catalyzed Asymmetric Intermolecular α-Arylation of Aldehydes with Aryl Bromides.
Angew. Chem. Int. Ed. 2021, 60, 18542–18546. (Hot Paper)
TY-Phos

210. Y. Wu, B. Xu, G. Zhao, Z. Pan, Z.-M. Zhang*, J. Zhang*.
Palladium/Xu-Phos Catalyzed Enantioselective Tandem Heck/Cacchi Reaction of Unactivated Alkenes.
Chin. J. Chem. 2021, 39, 3255–3260.
209. B. Yang, J. Yang*, J. Zhang*.
Synthesis of Axially Chiral Anilides Enabled by a Palladium/ Ming-Phos-Catalyzed Desymmetric Sonogashira Reaction.
Chin. J. Chem. 2022, 40, 317-322.
208. Zhenli Liu, Guanghui Li, Tengfei Yao, Junliang Zhang*, Lu Liu*.
Adv. Synth. Catal. 2021, 363, 2740-2745.

207. Qiaoyu Chen, Sanliang Li, Xiaoxiao Xie, Hao Guo*, Junfeng Yang*, and Junliang Zhang*
Org. Lett. 2021, 23, 4099–4103.
Xu-Phos

206. Wangqin Ji, Hai-Hong Wu, Wenbo Li* and Junliang Zhang*.
Copper-catalyzed cyclization reaction: synthesis of trifluoromethylated indolinyl ketones.
Chem. Commun., 2021, 57, 4448-4451.
205. Zhunzhun Yu, Guanghui Li, Junliang Zhang* and Lu Liu*.
Org. Chem. Front., 2021, 8, 3770-3775.
204. Yuzhuo Wang, Lei Wang,Mingjie Chen,Youshao Tu, Yu Liu* and Junliang Zhang*
Palladium/Xu-Phos-catalyzed asymmetric carboamination towards isoxazolidines and pyrrolidines.
Xu-Phos
2020年
203. Deyun Qian and Junliang Zhang*,
Yne–Enones Enable Diversity-Oriented Catalytic Cascade Reactions: A Rapid Assembly of Complexity.
Acc. Chem. Res. 2020, 53, 2358–2371.

Angew. Chem., Int. Ed. 2020, 59, 22957−22962.
TY-Phos

201. Chu, Haoke; Cheng, Jie; Yang, Junfeng; Guo, Yin-Long. Zhang Junliang*.
Asymmetric Dearomatization of Indole via Palladium/PC-Phos-Catalyzed Dynamic Kinetic Transformation.
Angew. Chem. Int. Ed. 2020, 59, 21991–21996. (Hot Paper)
PC-Phos

Angew. Chem. Int. Ed. 2020, 59, 2769–2775. (‡ These authors contributed equally to this work)
Xu-Phos

199. Dai, Qiang; Liu, Lu; Qian, Yanyan; Li, Wenbo; Zhang, Junliang*.
Angew.Chem. Int.Ed. 2020, 59, 20645–20650.
Xiao-Phos

198. Lei Wang, Kenan Zhang, Yuzhuo Wang, Wenbo Li, Mingjie Chen, and Junliang Zhang*.
Angew. Chem. Int. Ed. 2020, 59, 4421 – 4427.
Xiang-Phos

J. Am. Chem. Soc. 2020, 142, 9763-9771.
Ming-Phos

196. Wangqin Ji, Hai-Hong Wu,* and Junliang Zhang*,
ACS Catal. 2020, 10, 1548−1554.
WJ-Phos

195. Tao, Mengna; Tu, Youshao; Liu, Yu; Wu, Haihong; Liu, Lu*; Zhang, Junliang*.
Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization under mild conditions.
Chem. Sci., 2020, 11, 6283-6288.
Xiang-Phos
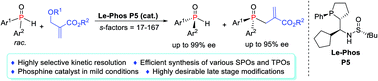
194. Qiu, Haile; Dai, Qiang; He, Jiafeng; Li, Wenbo; Zhang, Junliang*.
Access to P-chiral sec- and tert-phosphine oxides enabled by Le-Phos-catalyzed asymmetric kinetic resolution.
Chem. Sci., 2020, 11, 9983-9988.
Le-Phos
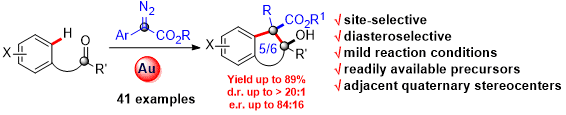
193. Lujia Zhou, Bing Xu, Danting Ji, Zhan-Ming Zhang,*and Junliang Zhang*,
Ming-Phos/Gold(I)-Catalyzed Stereodivergent Synthesis of HighlySubstituted Furo[3,4-d][1,2]oxazines.
Chin. J. Chem. 2020, 38, 577-582.
Ming-Phos

192. Yongfeng Li, Zhiqiong Tang, Junliang Zhang,* and Lu Liu*,
Chem. Commun., 2020, 56, 8202-8205.
191. Liu, Bing; Qiu, Haile; Chen, Xiaofeng; Li, Wenbo; Zhang, Junliang*.
Copper-catalyzed asymmetric tandem borylative addition and aldol cyclization.
Org. Chem. Front., 2020, 7, 2492-2498.
190. Li, Wenbo; Zhang, Junliang*.
Synthesis of Heterocycles through Denitrogenative Cyclization of Triazoles and Benzotriazoles.
Chem. Eur. J. 2020, 26, 11931-11945.

189. Ma, Ben; Tang, Zhiqiong; Zhang, Junliang*; Liu, Lu.*
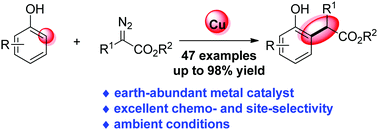
Copper-catalysed ortho-selective C-H bond functionalization of phenols and naphthols with α-aryl-α-diazoesters.
Chem. Commun., 2020, 56, 9485-9488.
188. Wang, Huamin; Li, Xiuzheng; Tu, Youshao; Zhang, Junliang*.
Catalytic Enantiodivergent Michael Addition by Subtle Adjustment of Achiral Amino Moiety of Dipeptide Phosphines. iScience 2020, 23, 101138.

187. Li, Sanliang; Chen, Qiaoyu; Li, Wenbo; Gu, Guanxin; Zhang, Junliang*.
Visible Light Driven Copper(I) Catalyzed Oxyamination of Electron Deficient Alkenes.
Chin. J. Chem. 2020, 38, 1116-1122.

186. Qi, Shutao; Gao, Shiquan; Xie, Xiaoxiao; Yang, Junfeng; Zhang, Junliang.
Org. Lett. 2020, 22, 5229-5234.

185. Yao, Tengfei; Zhang, Fang; Zhang, Junliang*; Liu, Lu*.
Palladium-Catalyzed Intermolecular Heck-Type Dearomative [4 + 2] Annulation of 2H-Isoindole Derivatives with Internal Alkynes.
Org. Lett. 2020, 22, 5063-5067.

184. Qian, Yanyan; Dai, Qiang; Li, Zhiming; Liu, Yu; Zhang, Junliang.
O-Phosphination of Aldehydes/Ketones toward Phosphoric Esters: Experimental and Mechanistic Studies.
Org. Lett. 2020, 22, 4742-4748.

183. Tengfei Yao, Tong Xia, Wei Yan, Haofeng Xu, Fang Zhang, Yuanjing Xiao, Junliang Zhang*and Lu Liu*
Copper-Catalyzed Chemodivergent Cyclization of N‑(orthoalkynyl)aryl-Pyrrole and Indoles.
Org. Lett. 2020, 22, 4511-4516.

2019年
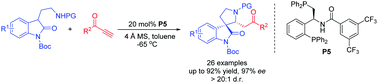
182. Dai, Qiang; Li, Wenbo; Li, Zhiming; Zhang, Junliang*.
J. Am. Chem. Soc. 2019, 141, 20556-20564.
Xiao-Phos

181. Zhu, Chenghao; Chu, Haoke; Li, Gen; Ma, Shengming;* Zhang, Junliang.*
Pd-Catalyzed Enantioselective Heck Reaction of Aryl Triflates and Alkynes.
J. Am. Chem. Soc. 2019, 141, 19246-19251.
Xu-Phos
(首次将亚磺酰胺膦配体统称Sadphos家族配体)

180. Zhang, Zhan-Ming; Xu, Bing; Wu, Lizuo; Wu, Yuanqi; Qian, Yanyan; Zhou, Lujia; Liu, Yu; Zhang, Junliang *
Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction
Angew. Chem. Int. Ed. 2019, 58, 14653–14659.
Xu-Phos

179. Pei-Chao Zhang, Jie Han and Junliang Zhang*
Angew. Chem. Int. Ed. 2019, 58, 11444-11448 (VIP).
PC-Phos

Palladium/XuPhos-Catalyzed Enantioselective Carboiodination of Olefin-Tethered Aryl Iodides
J. Am. Chem. Soc. 2019, 141, 8110-8115. (‡ These authors contributed equally to this work)
Xu-Phos

177. Huamin Wang‡, Junyou Zhang‡, Youshao Tu and Junliang Zhang*
Phosphine-Catalyzed Enantioselective Dearomative [3+2]-Cycloaddition of 3-Nitroindoles and 2-Nitrobenzofurans
Angew. Chem. Int. Ed. 2019, 58, 5422-5426.
(‡ These authors contributed equally to this work)
Xiao-Phos

176. Qiu, Haile;‡ Chen, Xiaofeng;‡ Zhang, Junliang.
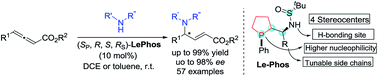
Design, synthesis and application of a new type of bifunctional Le-Phos in highly enantioselective γ-addition reactions of N-centered nucleophiles to allenoates
Chem. Sci., 2019,10, 10510-10515.
(‡ These authors contributed equally to this work)
Le-Phos
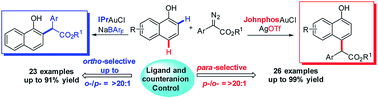
175. Yu, Zhunzhun; Li, Yongfeng; Zhang, Pei-Chao; Liu, Lu; Zhang, Junliang.
Ligand and counteranion enabled regiodivergent C–H bond functionalization of naphthols with α-aryl-α-diazoesters
Chem. Sci., 2019,10, 6553-6559.
174. Xiaoyu Di, Yidong Wang, Lizuo Wu, Zhan-Ming Zhang, Qiang Dai, Wenbo Li,* Junliang Zhang*.
Enantioselective Gold(I)-Catalyzed Heterocyclization–Intermolecular Exo [4 + 3]-Cycloaddition Reactions for the Synthesis of Chiral Oxa-Bridged Benzocycloheptanes.
Org. Lett. 2019, 21, 3018−3022.
Ming-Phos

173. Han, Jie; Zhou, Wei; Zhang, Pei-Chao; Wang, Huamin; Zhang, Ronghua; Wu, Hai-Hong; Zhang, Junliang.
Design and Synthesis of WJ-Phos, and Application in Cu-Catalyzed Enantioselective Boroacylation of 1,1-Disubstituted Allenes.
ACS Catal. 2019, 9, 6890-6895.
WJ-Phos

172. Zhaowei Chu, Zhiqiong Tang, Kenan Zhang, Lei Wang, Wenbo Li,* Hai-Hong Wu,* and Junliang Zhang*
Gold(I)-Catalyzed Enantioselective Cyclopropanation of α-Aryl Diazoacetates with Enamides.
Organometallics, 2019, 38, 4036−4042.

171. Liu, Zhenli; Xu, Haofeng; Yao, Tengfei; Zhang, Junliang;* Liu, Lu.*
Catalyst-Enabled Chemodivergent Construction of Alkynyl- and Vinyl-Substituted Diarylmethanes from p-Quinone Methides and Alkynes.
Org. Lett. 2019, 21, 7539-7543.

170. Cong, Tiantian; Wang, Huamin; Li, Xiuzheng; Wu, Hai-Hong; Zhang, Junliang*
Chiral bifunctional bisphosphine enabled enantioselective tandem Michael addition of tryptamine-derived oxindoles to ynones.
Chem. Commun., 2019, 55, 9176-9179.
Peng-Phos
169. Chenghao Zhu, Junliang Zhang*
Nickel-catalyzed alkyl–alkyl cross-coupling reactions of non-activated secondary alkyl bromides with aldehydes as alkyl carbanion equivalents.
Chem. Commun., 2019, 55, 2793-2796.
168. Bing Liu, Wenbo Li, Hai-Hong Wu *, Junliang Zhang*.
Enantiodivergent synthesis of 1,2-bis(diphenylphosphino)ethanes via asymmetric [3 + 2]-cycloaddition.
Org. Chem. Front., 2019, 6, 694–698.
Ruthenium(II)-cored supramolecular organic framework-mediated recyclable visible light photoreduction of azides to amines and cascade formation of lactams.
Chin. Chem. Lett., 2019, 30, 1383-1386.

166. Lei Wang, Mingjie Chen, Junliang Zhang*.
Transition metal-free base-promoted arylation of sulfenate anions with diaryliodonium salts.
Org. Chem. Front., 2019, 6, 32–35.
2018年
165. Huamin Wang, Li Zhang, Youshao Tu, Ruiqi Xiang, Yin-Long Guo* and Junliang Zhang*
Phosphine-Catalyzed Difunctionalization of β-Fluoroalkyl α,β-Enones: A Direct Approach to β-Amino α-Diazo Carbonyl Compounds.
Angew. Chem. Int. Ed. 2018, 57, 15787-15791.
Peng-Phos

164. Zhan-Ming Zhang+, Bing Xu+, Yanyan Qian, Lizuo Wu, Yuanqi Wu, Lujia Zhou, Yu Liu and Junliang Zhang*
Palladium-Catalyzed Enantioselective Reductive Heck Reactions: Convenient Access to 3,3-Disubstituted 2,3-Dihydrobenzofuran.
Angew. Chem. Int. Ed. 2018, 57, 10373-10377. (‡ These authors contributed equally to this work)
Xu-Phos

163. Lei Wang, Mingjie Chen, Pei-Chao Zhang, Wenbo Li and Junliang Zhang*
Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General Sulfenate Anions: Scope and Synthetic Applications.
J. Am. Chem. Soc. 2018, 140, 3467-3473.
PC-Phos

162. Shan Xu, Zhan-Ming Zhang, Bing Xu, Bing Liu, Yuanyuan Liu,* and Junliang Zhang*
Enantioselective Regiodivergent Synthesis of Chiral Pyrrolidines with Two Quaternary Stereocenters via Ligand-Controlled Copper(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloadditions.
J. Am. Chem. Soc. 2018, 140, 2272-2283.

161. Tao-Yan Lin, Hai-Hong Wu, Jian-Jun Feng, Junliang Zhang*
Chirality Transfer in Rhodium(I)-Catalyzed [3 + 2]-Cycloaddition of Vinyl Aziridines and Oxime Ethers: Atom-Economical Synthesis of Chiral Imidazolidines.
Org. Lett., 2018, 20 , 3587-3590.

160. Bing Xu, Zhan-Ming Zhang, Lujia Zhou, Junliang Zhang*
Direct Asymmetric Formal [3 + 2] Cycloaddition Reaction of Isocyanoesters with β-Trifluoromethyl β,β-Disubstituted Enones Leading to Optically Active Dihydropyrroles.
Org. Lett., 2018, 20, 2716-2719.

159. Bing Liu, Zhan-Ming Zhang, Bing Xu, Shan Xu, Hai-Hong Wu, Junliang Zhang*
Cu(I)-Ming-phos Catalyzed Enantioselective [3+2] Cycloadditions of Glycine ketimines to β-Trifluoromethyl Enones.
Adv. Synth. Catal., 2018, 360, 2144-2150.
Ming-Phos

158. Mei Zhang, Xiaoyu Di, Mingrui Zhang, Junliang Zhang*
Gold(I)-Catalyzed Diastereo- and Enantioselective Synthesis of Polysubstituted Pyrrolo[3,4-d][1,2]oxazines.
Chin. J. Chem, 2018, 36, 519-525.

157. Shan Xu, Bing Liu, Zhan-Ming Zhang, Bing Xu, Junliang Zhang*
Copper(I)-Catalyzed Asymmetric [3+2]-Cycloaddition of α-Substituted Iminoesters with α-Trifluoromethyl α,β-Unsaturated Esters.
Chin. J. Chem. 2018, 36, 421-429.

156. Chao-Ze Zhu, Jian-Jun Feng, Junliang Zhang*
Divergent synthesis of functionalized pyrrolidines and γ-amino ketones via rhodium-catalyzed switchable reactions of vinyl aziridines and silyl enol ethers.
Chem. Commun., 2018, 54, 2401-2404.
155. Qin Zeng, Li Zhang, Yuanjing Xiao,* Junliang Zhang*
Divergent access to N-hydroxypyrroles and isoxazoles via the gold(i)- or Brønsted acid-catalysed regioselective cyclization of N-(2-trifluoromethyl-3-alkynyl) oximes.
Org. Biomol. Chem, 2018, 16, 1375-1380.
154. Qiang Dai, Junliang Zhang*
Direct Synthesis of Sulfinamides by the Copper-Catalyzed Electrophilic Amidation of Sulfenate Anions.
Adv. Synth. Catal., 2018, 360, 1123-1127.

153. Peng Chen, Junyou Zhang, Junliang Zhang*
Highly Substituted Cyclohexenes via Phosphine-Catalyzed [4+2] Annulation of Electron-deficient Dienes and Vinyl Ketones.
Adv. Synth. Catal., 2018, 360, 682-685.

152. Pei-Chao Zhang, Yidong Wang, Zhan-Ming Zhang, Junliang Zhang*
Gold(I)/Xiang-Phos-Catalyzed Asymmetric Intramolecular Cyclopropanation of Indenes and Trisubstituted Alkenes.
Org. Lett, 2018, 20, 7049-7052.
Xiang-Phos

151. Tao Zhou, Tong Xia, Zhenli Liu, Lu Liu,* Junliang Zhang*
Asymmetric Phosphine-Catalyzed [4+1] Annulations of o-Quinone Methides with MBH Carbonates.
Adv. Synth. Catal.,2018, 360, 4475-4479.

150. Huayu Cheng, Xiaofan Zhou, Anjing Hu, Shiteng Ding, Yimo Wang, Yuanjing Xiao,* Junliang Zhang*
Thioether-functionalized trifluoromethyl-alkynes, 1,3-dienes and allenes: divergent synthesis from reaction of 2-trifluoromethyl-1,3-conjugated enynes with sulfur nucleophiles.
RSC .Adv. 2018, 8, 34088-34093.
149. Yongfeng Li, Huamin Wang, Yuwei Su, Runchen Li, Cao Li, Lu Liu,* Junliang Zhang*
Phosphine-Catalyzed [3 + 2] Cycloaddition Reaction of α-Diazoacetates and β-Trifluoromethyl Enones: A Facile Access to Multisubstituted 4-(Trifluoromethyl)pyrazolines.
Org. Lett. 2018, 20, 6444-6448.

148. Yidong Wang, Zhan-Ming Zhang, Feng Liu, Yinyan He, Junliang Zhang*
Ming-Phos/Gold(I)-Catalyzed Diastereo- and Enantioselective Synthesis of Indolyl-Substituted Cyclopenta[c]furans.
Org. Lett. 2018, 20, 6403-6406.
Ming-Phos

147. Ben Ma, Lu Liu,* Junliang Zhang*
Gold-Catalyzed Site-Selective C−H Bond Functionalization with Diazo Compounds.
Asian J. Org. Chem., 2018, 7, 2015-2025.

146. Bing Liu, Hai-Hong Wu, Junliang Zhang*
Cu(II)-Catalyzed Enantioselective β-Boration of β-Trifluoromethyl, β,β-Disubstituted Enones and Esters: Construction of a CF3- and Boron-Containing Quaternary Stereocenter.
ACS .Catal., 2018, 8, 8318-8323.

2017年
145. Yidong Wang,‡ Pei-Chao Zhang,‡ Xiaoyu Di, Qiang Dai, Zhan-Ming Zhang and Junliang Zhang*
Gold-Catalyzed Asymmetric Intramolecular Cyclization of N-Allenamides for the Synthesis of Chiral Tetrahydrocarbolines
Angew. Chem. Int. Ed. 2017, 56, 15905-15909. (‡ These authors contributed equally to this work)
PC-Phos

144. Ben Ma, Zhaowei Chu, Ben Huang, Zhenli Liu, Lu Liu* and Junliang Zhang*
Highly para-Selective C−H Alkylation of Benzene Derivatives with 2,2,2-Trifluoroethyl α-Aryl-α-Diazoesters
Angew. Chem. Int. Ed., 2017, 56, 2749-2753.

143. Chao-Ze Zhu, Jian-Jun Feng,* and Junliang Zhang*
Rhodium(I)-Catalyzed Intermolecular Aza-[4+3] Cycloaddition of Vinyl Aziridines and Dienes: Atom-Economical Synthesis of Enantiomerically Enriched Functionalized Azepines.
Angew. Chem. Int. Ed. 2017, 56, 1351-1355.

142. Yangyan Li, WenboLi and Junliang Zhang*
Gold-Catalyzed Enantioselective Annulations.
Chem. Eur. J., 2017, 23, 467–512.
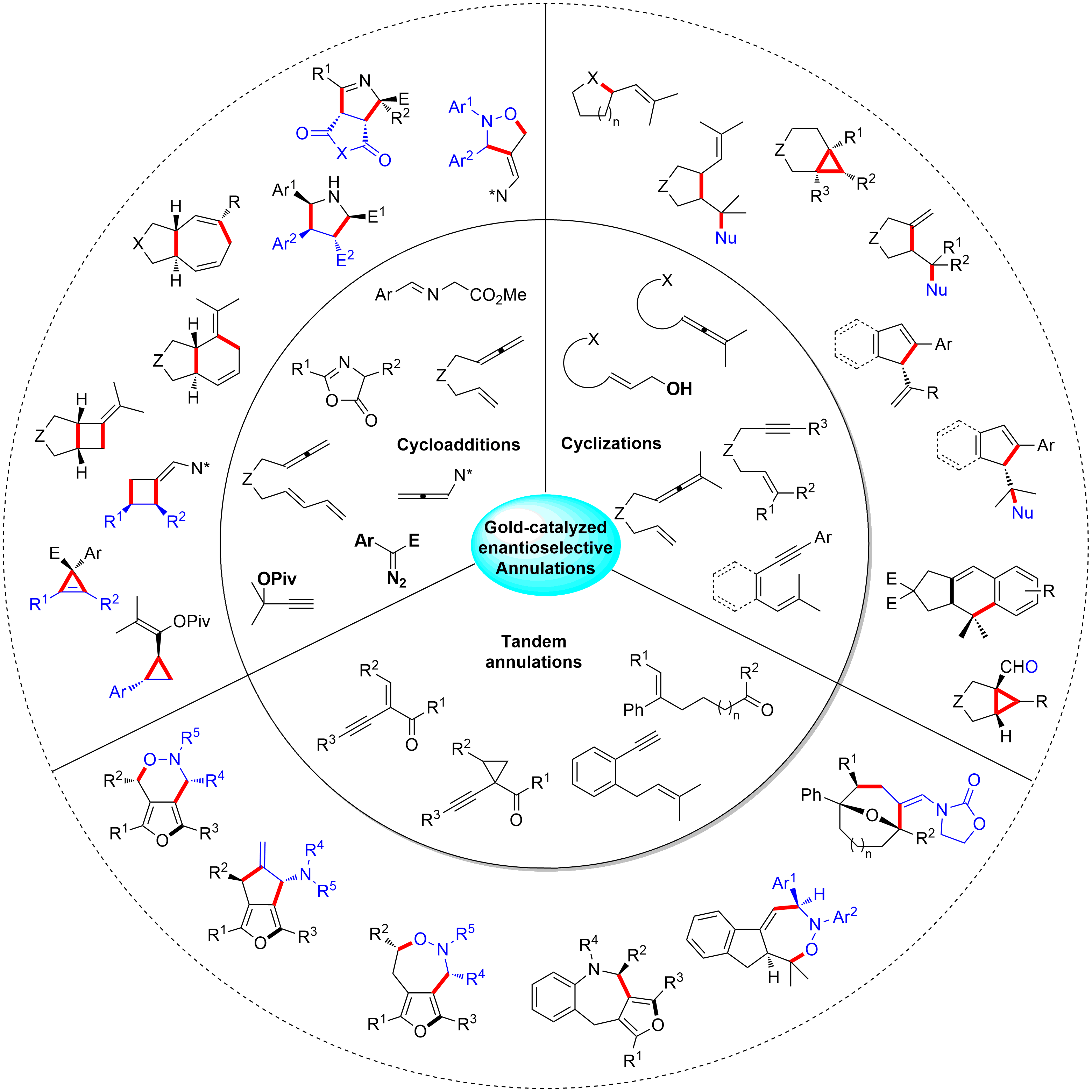
141. Bing Xu,‡ Zhan-Ming Zhang,‡ Shan Xu, Bing Liu, Yuanjing Xiao and Junliang Zhang*.
ACS Catal. 2017, 7, 210−214. (‡ These authors contributed equally to this work)
Ming-Phos

140. Xiaofan Zhou,Chaoqian Huang, Yu Zeng, Jiarui Xiong, Yuanjing Xiao*, Junliang Zhang*
Chem. Commun. 2017,53, 1084-1087.
139. Jieru Yang, Xiaofan Zhou,Yu Zeng, Chaoqian Huang, Yuanjing Xiao*, Junliang Zhang*
Org. Biomol. Chem., 2017, 15, 2253–2258.
138. Huamin Wang, Wei Zhou, Mengna Tao, Anjing Hu, Junliang Zhang*
Org. Lett. 2017, 19, 1710 – 1713.
Peng-Phos

137. Shenhuan Li, Yuanyuan Liu, Ben Huang, Tao Zhou, Hongmei Tao, Yuanjing Xiao, Lu Liu*, Junliang Zhang* Phosphine-Catalyzed Asymmetric Intermolecular Cross-Vinylogous Rauhut–Currier Reactions of Vinyl Ketones with para-Quinone Methides.
ACS. Catal. 2017,7, 2805 – 2809.
Peng-Phos

136. Taoyan Lin,Haihong Wu, Jianjun Feng, Junliang Zhang*
Org. Lett. 2017, 19, 2897-2900.

135. Taoyan Lin, Haihong Wu, Jianjun Feng*, Junliang Zhang*
ACS. Catal. 2017, 7, 4047 – 4052.

134. Pei-Chao Zhang, Yidong Wang, Deyun Qian, Wenbo Li*, Junliang Zhang*
Synthesis of Substituted Naphtho-Ferrocenes via a Gold(I)-Catalyzed Intramolecular 6-endo-Dig Cyclization.
Chin. J. Chem. 2017,35, 849 – 852.

133. Xingxing Wu, Wei Zhou, Haihong Wu, Junliang Zhang*
Chem. Commun. 2017, 53, 5661 – 5664.
132. Chaoze Zhu, Jianjun Feng*, Junliang Zhang*
Chem. Commun. 2017 , 53, 4688 – 4691.
131. Wei Zhou, HuaminWang, Mengna Tao, Chaoze Zhu, Taoyan Lin, Junliang Zhang*
Chem. Sci. 2017, 8, 4660 – 4665.
130. Bing Xu, Zhanmin Zhang, Bing Liu, Shan Xu, Liejin Zhou, Junliang Zhang*
Chem. Commun. 2017, 53, 8152 – 8155.
129. Lu Liu, Junliang Zhang*
过渡金属催化重氮化合物参与的芳烃碳(sp2)-氢键官能团化研究进展.
Chin. J. Org. Chem. 2017, 37, 1117 – 1126.

128. Jianjun Feng*, Junliang Zhang*
ACS. Catal. 2017, 7, 1533 – 1542.

127. Yuanyuan Liu, Wenbo Li, Junliang Zhang*
Chiral ligands designed in China.
Natl. Sci. Rev. 2017, 4, 326 – 358.

126. Bing Liu, Zhanmin Zhang, Bing Xu, Shan Xu, Haihong Wu, Yuanyuan Liu*, Junliang Zhang*
Org. Chem. Front. 2017, 4, 1772 – 1776.
125. Yu Zeng, Chaoqian Huang, PeiyunNi, Lu Liu, Yuanjing Xiao*, Junliang Zhang*
Adv. Synth. Catal. 2017, 359, 3555 – 3559.

124. Ben Ma, Jiaojiao Wu, Lu Liu*, Junliang Zhang*
Chem. Commun. 2017 ,53, 10164 – 10167.
123. Chaoqian Huang, Yu Zeng, Huayu Cheng, Anjing Hu, Lu Liu, Yuanjing Xiao*, Junliang Zhang*
Org. Lett. 2017,19, 4968 – 4971.

122. MengnaTao, Wei Zhou, Junliang Zhang*
Adv. Synth. Catal. 2017, 359, 3347 – 3353.
Peng-Phos

121. Huamin Wang, Weike Lu, Junliang Zhang*
Chem. Eur. J. 2017, 23, 13587 – 13590.

Org. Biomol. Chem., 2017, 15, 4941–4945.
119. Ben Huang, Chao Li, Huamin Wang, Caihui Wang, Lu Liu*, Junliang Zhang*
Org. Lett. 2017, 19, 5102−5105.

118. Taoyan Lin, Haihong Wu, Jianjun Feng*, Junliang Zhang*
Org. Lett. 2017, 19, 6526–6529.

117. Junyou Zhang, Haihong Wu*, Junliang Zhang*
Enantioselective Phosphine-Catalyzed Allylic Alkylations of mix-Indene with MBH Carbonates.
Org. Lett. 2017, 19, 6080−6083.
Xiao-Phos

116. Chaoze Zhu, Jianjun Feng,* junliang Zhang*
铑催化烯基氮杂环丙烷与炔胺的立体专一性[3+2]环加成反应(封面文章)
Chin. J. Org. Chem. 2017, 37, 1165-1172.

115. Haoxiang Hu, Yidong Wang, Deyun Qian, Zhan-Ming Zhang, Lu Liu* and Junliang Zhang*
Org. Chem. Front., 2016,3, 759-763.
Xiang-Phos
114. W. Zhou, L. Gao, M. Tao, X. Su, Q. Zhao, J. Zhang*
多功能手性膦催化活泼烯烃的不对称分子间Rauhut-Currier反应.
Acta Chim. Sinica 2016, 74, 800-804.
Xiao-Phos

113. W. Zhou, Z. Yue, J. Zhang*
A highly efficient one-pot trifluoromethylation/cyclization reaction of electron-deficient 1,3-conjugated enynes: modular access to trifluoromethylated furans and 2,3-dihydrofurans.
Org. Chem. Front. 2016, 3, 1416.
112. Zhunzhun Yu,Yongfeng Li, Jiameng Shi, Ben Ma, Lu Liu and Junliang Zhang*
(C6F5)3B -Catalyzed Highly Chemoselective and Ortho-selective C-H Bond Functionalization of Phenols Oriented by Hydrogen Bonds.
Angew. Chem. Int. Ed., 2016, 55, 14807-14811.
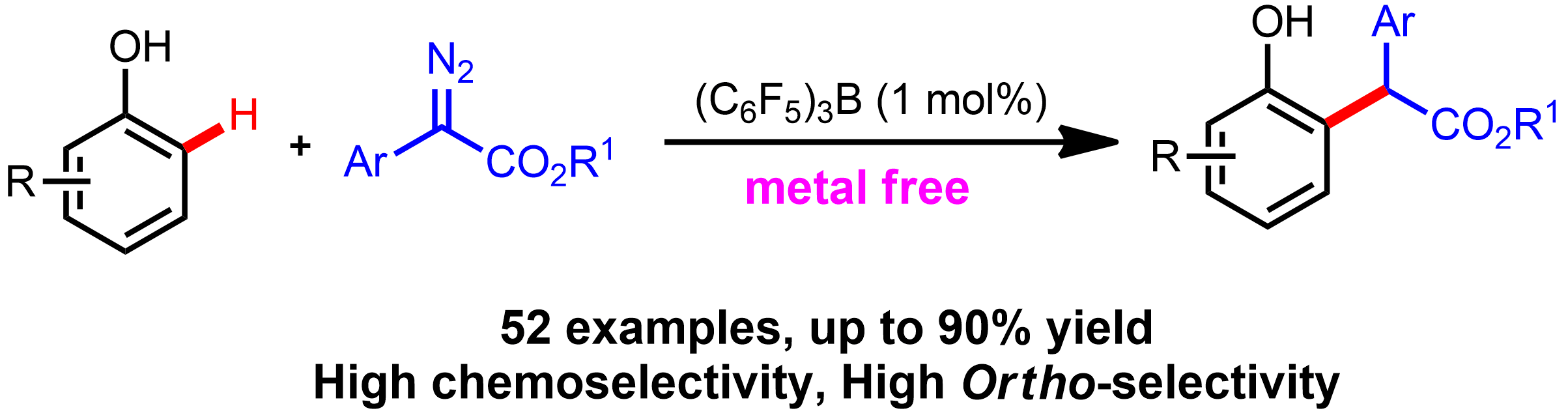
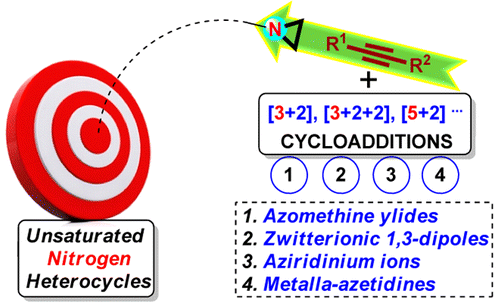
111. Feng, Jian-Jun;* Zhang, Junliang*
Synthesis of Unsaturated N-Heterocycles by Cycloadditions of Aziridines and Alkynes.
ACS Catal. 2016, 6, 6651-6661. (Invited Review)
110. Peng Chen, Zhenting Yue, Junyou Zhang, Xi Lv, Lei Wang and Junliang Zhang*
Phosphine-Catalyzed Asymmetric Umpolung Addition of Trifluoromethyl Ketimines to Morita–Baylis–Hillman Carbonates.
Angew. Chem. Int. Ed. 2016, 55, 13316-13320.
Peng-Phos

109. Zhenting Yue, Wenbo Li, Lu Liu, Cuihong Wang* and Junliang Zhang*
Enantioselective Synthesis of 4H-Pyrans Through Organocatalytic Asymmetric Formal [3+3] Cycloadditions of 2-(1-Alkynyl)-2-alken-1-ones with β-Keto Esters.
Adv. Synth. Catal. 2016, 358, 3015-3020 (VIP).

108. Lin, Tao-Yan; Zhu, Chao-Ze; Zhang, Pei-Chao; Wang, Yidong; Wu, Hai-Hong; Feng, Jian-Jun;* Zhang, Junliang*
Regiodivergent Intermolecular [3+2] Cycloadditions of Vinyl Aziridines and Allenes: Stereospecific Synthesis of Chiral Pyrrolidines.
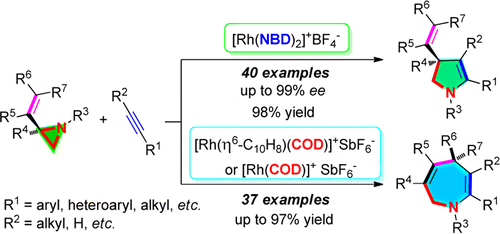
Angew. Chem. Int. Ed. 2016, 55, 10844-10848.
107. WenboLi, Xiuzhao Yu, Zhenting Yue, and Junliang Zhang*
Asymmetric Construction of 2,3-Dihydroisoxazoles via an Organocatalytic Formal [3 + 2] Cycloaddition of Enynes withN-Hydroxylamines.
Org. Lett. 2016, 18, 3972–3975
106. Ben Ma, Ziang Wu, Ben Huang, Lu Liu* and Junliang Zhang*
Gold-catalysed facile access to indene scaffold via sequential C-H functionalization and 5-endo-dig carbocyclization.
Chem. Commun, 2016, 52, 9351-9354.
105. Wei Zhou, Peng Chen, Mengna Tao, Xiao Su, Qingjie Zhao* and Junliang Zhang*
Enantioselective intermolecular cross Rauhut–Currier reactions of activated alkenes with acrolein.
Chem. Commun, 2016, 52, 7612-7615.
Xiao-Phos
104. ZhunZhun Yu, Lu Liu* and Junliang Zhang*
Triflic Acid-Catalyzed Enynes Cyclization: A New Strategy beyond Electrophilic π-Activation.
Chem. Eur. J., 2016, 22, 8488-8492.

103. Zhan-Ming Zhang,‡ Bing Xu,‡ Shan Xu, Hai-Hong Wu and Junliang Zhang*
Diastereo- and Enantioselective Copper(I)-Catalyzed Intermolecular [3+2] Cycloaddition of Azomethine Ylides with β-Trifluoromethyl β,β-Disubstituted Enones.
Angew. Chem. Int. Ed. 2016, 55, 6324–6328. (‡ These authors contributed equally to this work)
Ming-Phos

102. Jieru Yang, Xiaofan Zhou, Yu Zeng, Chaoqian Huang, Yuanjing Xiao* and Junliang Zhang*
Synthesis of 2-fluoro-2-pyrrolines via tandem reaction of α-trifluoromethyl-α,β-unsaturated carbonyl compounds with N-tosylated 2-aminomalonates.
Chem. Commun., 2016, 52, 4922.
101. Jian-Jun Feng,‡ Tao-Yan Lin,‡ Chao-Ze Zhu, Huamin Wang, Hai-Hong Wu and Junliang Zhang*.
The Divergent Synthesis of Nitrogen Heterocycles by Rhodium(I)-Catalyzed Intermolecular Cycloadditions of Vinyl Aziridines and Alkynes.
J. Am. Chem. Soc. 2016, 138, 2178-2181. (‡ These authors contributed equally to this work)
100. Wenbo Li and Junliang Zhang*
Recent developments in the synthesis and utilization of chiral β-aminophosphine derivatives as catalysts or ligands.
Chem. Soc. Rev. 2016, 45, 1657-1677.
第100篇文章

99. Peng Chen, Xiao Su, Wei Zhou, Yuanjing Xiao* and Junliang Zhang*
Novel Chiral Sulfinamide Phosphines : Valuable Precursors to Chiral β-Aminophosphines.
Tetrahedron 2016, 72, 2700-2706.
Xiao-Phos

98.Yanqing Zhang, Yuanjing Xiao* and Junliang Zhang*
Gold(I)-Catalyzed Stereospecific [4+3]-Cycloaddition Reaction of 1-(Alk-1-ynyl)cyclopropyl Ketones with Nitrones: A Modular Entry to Enantioenriched 5,7-Fused Bicyclic Furo[3,4-d][1,2]oxazepines.
Synthesis 2016, 48, 512-519. (Invited feature article)

97. Lu Liu* and Junliang Zhang*
Gold-catalyzed transformations of α-diazocarbonyl compounds: selectivity and diversity.
Chem. Soc. Rev. 2016, 45, 506-516.

96. Zhunzhun Yu, Haile Qiu, Lu Liu* and Junliang Zhang*
Gold-Catalyzed Construction of Two Adjacent Quaternary Stereocenters via Sequential C-H Functionalization and Aldol Annulation.
Chem. Commun. 2016, 52, 2257-2260.
95 Wenbo Li, Liejing Zhou and Junliang Zhang*
Recent Progress in Dehydro(genative) Diels–Alder Reaction.
Chem. Eur. J. 2016, 22, 1558-1571. (Invited Reviews)

94. Yuan Liu,‡ Zhunzhun Yu,‡ John Zenghui Zhang, Lu Liu,* Fei Xia* and Junliang Zhang*
Origins of Unique Gold-Catalyzed Chemo- and Site-Selective C-H Functionalization of Phenols with Diazo Compounds.
Chem. Sci. 2016,7, 1988-1995. (‡These authors contributed equally to this work)
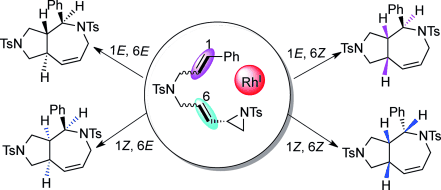
93 Jian-Jun Feng, Tao-Yan Lin, Hai-Hong Wu, and Junliang Zhang*
Modular Access to the Stereoisomers of Fused Bicyclic Azepines: Rhodium-Catalyzed Intramolecular Stereospecific Hetero-[5+2] Cycloaddition of Vinyl Aziridines and Alkenes.
Angew. Chem. Int. Ed. 2015, 54, 15854-15858.
92. Mingjin Chen, Zhan-Ming Zhang, Zhunzhun Yu, Haile Qiu, Ben Ma, Hai-Hong Wu* and Junliang Zhang*
Polymer-Bound Chiral Gold-Based Complexes as Efficient Heterogeneous Catalysts for Enantioselectivity Tunable Cycloaddition.
ACS Catal. 2015, 5, 7488−7492.
Ming-Phos
91. Wenbo Li, Lihua Gao, Zhenting Yue and Junliang Zhang*
Phosphine-Catalyzed Regioselective and Stereoselective Hydrohalogenation Reaction of 2-(1-Alkynyl)-2-alken-1-ones: Synthesis of 2-Halo-1,3-dienes.
Adv. Synth. Catal. 2015, 357, 2651-2655.

90. Wei Zhou, Xiao Su, Mengna Tao,Chaoze Zhu, Qingjie Zhao* and Junliang Zhang*
A Novel Chiral Sulfinamide Bisphosphine Catalysts: Design, Synthesis, and Application in Highly Enantioselective Inter-molecular Cross Rauhut–Currier Reactions.
Angew. Chem. Int. Ed. 2015, 54, 14853-14857.
Wei-Phos
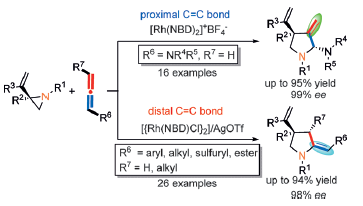
89. Yidong Wang, Pei-Chao Zhang, Deyun Qian and Junliang Zhang*
Highly Regio-, Diastereo-, and Enantioselective Gold(I)-Catalyzed Intermolecular Annulations with N-Allenamides at the Proximal C=C Bond.
Angew. Chem. Int. Ed. 2015, 54, 14849-14852 (Cover paper).
(Highlighted by Synfacts 2015, 11, 1302)
88. Yidong Wang, Peichao Zhang, Yuan Liu, Fei Xia* and Junliang Zhang*
Enantioselective Gold-Catalyzed Intermolecular [2+2] versus [4+2]-Cycloadditions of 3-Styrylindoles with N-Allenamides: Oberservation of Interesting Substituent Effect.
Chem. Sci. 2015, 6, 5564-5570.
(Highlighted by Synfacts 2016, 12, 55)
87. Yangyan Li, Xiao Su, Wei Zhou, Wenbo Li and Junliang Zhang*
Amino Acid Derived Phosphine-Catalyzed Enantioselective 1,4-Dipolar Spiroannulation of Cyclobutenones and Isatylidenemalononitrile.
Chem. Eur. J. 2015, 21, 4224-4228.

86. Liejin Zhou, Bing Xu and Junliang Zhang*
Metal-free Dehydrogenative Diels-Alder Reactions of 2-Methyl, 3-Aryl-methyl-indoles and Dienophiles:A Rapid Access to Tetrahydrocarbazoles, Carbazoles and Heteroacenes.
Angew. Chem. Int. Ed.2015, 54, 9092-9096.
85. Feng Liu, Yidong Wang, Weiming Ye, and Junliang Zhang*
Gold(I)-catalyzed asymmetric [3 + 2]-cycloadditions of γ-1-ethoxyethoxy-propiolates and aldehydes.
Org. Chem. Front.2015, 2, 221-225.
84. Xiao Su, Wei Zhou, Yangyan Li, and Junliang Zhang*
Design, Synthesis, and Application of a Chiral Sulfinamide Phosphine Catalyst for the Enantioselective Intramolecular Rauhut–Currier Reaction.
Angew. Chem. Int. Ed. 2015, 54, 6874-6877.
Xiao-Phos
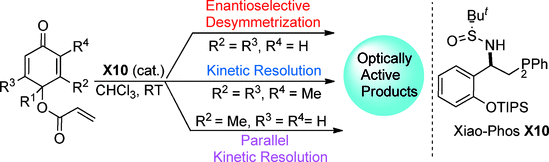
83. Jieru Yang, Ao Mao, Zhenting Yue, Wenxuan Zhu, Xuewei Luo, Chuwei Zhu, Yuanjing Xiao* and Junliang Zhang*
A simple base-mediated synthesis of diverse functionalized ring-fluorinated 4H-pyrans via double direct C–F substitutions.
Chem. Commun.2015, 51, 8326-8329. (Highlighted by http://x-mol.com/news/508)
82. Jian-Jun Feng, Tao-Yan Lin, Hai-Hong Wu and Junliang Zhang*
Transfer of Chirality in the Rhodium-Catalyzed Intramolecular Formal Hetero-[5 + 2] Cycloaddition of Vinyl Aziridines and Alkynes: Stereoselective Synthesis of Fused Azepine Derivatives.
J. Am.Chem. Soc. 2015, 137, 3787-3790.
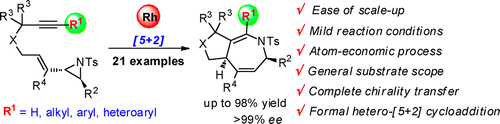
81. Deyun Qian and Junliang Zhang*
Gold-Catalyzed Cyclopropanation Reactions by Carbenoid Precursor Toolbox.
Chem. Soc. Rev. 2015, 44, 677-698. (Cover paper)
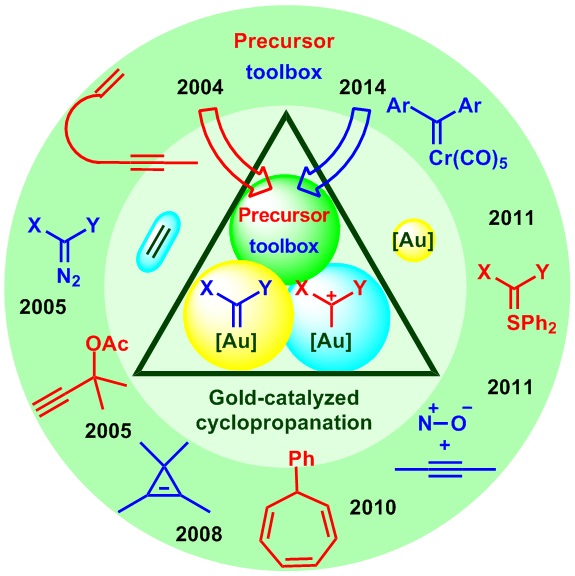
2014年
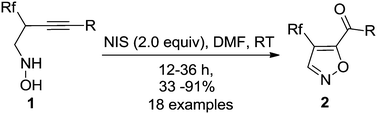
80. Li Zhang,Qin Zeng, Ao Mao, Ziang Wu, Tian Luo, Yuanjing Xiao* and Junliang Zhang*
NIS-mediated oxidative cyclization of N-(2-trifluoromethyl-3-alkynyl) hydroxylamines: a facile access to 4-trifluoromethyl-5-acylisoxazoles.
Org. Biomol. Chem.2014,12, 8942-8946.
79. Deyun Qian, Haoxiang Hu, Feng Liu, Bin Tang, Weimin Ye, Yidong Wang and Junliang Zhang*
Angew. Chem. Int. Ed. 2014, 53, 13751-13755.
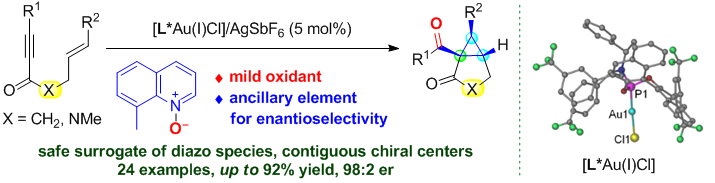
78. Lu Liu and Junliang Zhang*
Product Selectivity Control in the Domino Cyclization of 2-(2-Alkynylarylidene)-1,3-dicarbonyl Compounds Catalyzed by Metal Lewis Acids.
Synthesis, 2014, 46, 2133-2142. (Invited feature article)

77. Lai Wei, Lu Liu* and Junliang Zhang*
Scandium-Catalyzed Tandem Selective Oxirane Ring-opening / Friedel-Crafts Alkylation: A Facile Access to [1,4]Oxazino[4,3-a]indoles and 3,4-Dihydro-1H-pyrrolo[2,1-c][1,4]oxazines.
Org. Biomol. Chem.2014,12, 6869-6877.
76. Weimin Ye, Wenbo Li and Junliang Zhang*
A metal-free dyotropic-like rearrangement of 2-oxa allylic alcohols in the presence of organoboronic acids.
Chem. Commun., 2014,50, 9879-9882.
75. Liejin Zhou, Mingrui Zhang, Wenbo Li and Junliang Zhang*
Angew. Chem. Int. Ed. 2014, 53, 6542-6545.
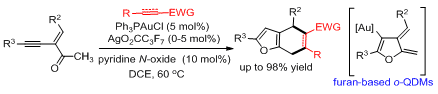
74. Zhunzhun Yu, Ben Ma, Mingjin Chen, Hai-Hong Wu, Lu Liu* and Junliang Zhang*
J. Am. Chem. Soc. 2014, 136, 6904-6907.
(Featured in JACS Spotlights:J. Am. Chem. Soc. 2014, 136, 7187−7188 当月JACS Most Read Articles top 3,同时被SCIENCE CHINA Chemistry 2014, 57, 1057重点评述)
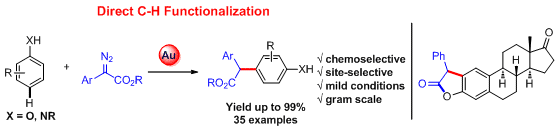
73. Deyun Qian and Junliang Zhang*
Gold-Catalyzed Cascade Reactions for Synthesis of Carbo- and Heterocycles: Selectivity and Diversity.
Chem. Rec. 2014, 14, 280-302. (Invited Account)
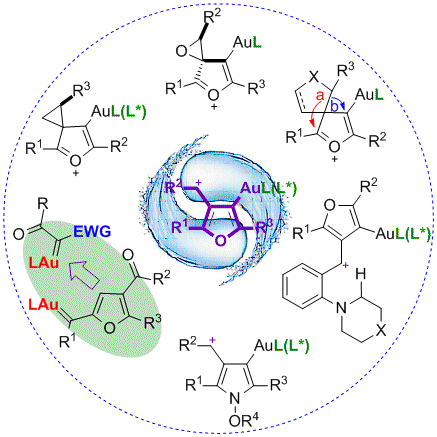
72. Zhan-Ming Zhang, Peng Chen, Wenbo Li, Yanfei Niu, Xiao-Li Zhao and Junliang Zhang*
Angew. Chem. Int. Ed. 2014, 53, 4350-4354.
First report of Sadphos: Ming-Phos

71. Ziqi Tian, Yuanjing Xiao, Xiangai Yuan, Zuliang Chen, Junliang Zhang*, and Jing Ma*
Control of Chemoselectivity by Coordinated Water and Relative Size of Ligands to Metal Cations of Lewis Acid Catalysts for Cycloaddition of an Oxirane Derivative to an Aldehyde: Theoretical and Experimental Study.
Organometallics 2014, 33, 1715-1725.

70. Qin Zeng, Li Zhang, Jieru Yang, Bing Xu, Yuanjing Xiao*and Junliang Zhang*
Pyrroles versus cyclic nitrones: catalyst-controlled divergent cyclization of N-(2-perfluoroalkyl-3-alkynyl) hydroxylamines.
Chem. Commun. 2014, 50, 4203-4206.
69. Wenbo Li and Junliang Zhang*
Phosphine-Mediated Regio- and Stereoselective Hydrocarboxylation of Enynes.
Org. Lett. 2014, 16, 162-165.

68. Mingrui Zhang and Junliang Zhang*
Base-Catalyzed Tandem Michael/Dehydro-Diels-Alder Reaction of α,α-Dicyanoolefins with Electron-Deficient 1,3-Conjugated Enynes: A Facile Entry to Angularly Fused Polycycles.
Chem. Eur. J. 2014, 20, 399-404.

2013年
67. Hui Qian, Xiuzhao Yu, Junliang Zhang* and Jianwei Sun*
J. Am. Chem. Soc. 2013,135, 18020–18023.
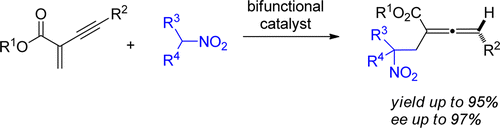
66. Mei Zhangand Junliang Zhang*
Gold(I)-Catalyzed Diastereoselective Domino Reactions of 2-(1-Alkynyl)alk-2-en-1-one Oxime Ethers with α,β-Unsaturated Imines Consisting of 1,2-Alkyl Migration.
Isr. J. Chem. 2013, 53, 911–914.

65. Junqing Zhang, Yuanjing Xiao and Junliang Zhang*
Nickel-Catalyzed Annulation of Donor–Acceptor Oxiranes with Imines: Diastereoselective Access to Highly Substituted 2,4-trans-Oxazolidines.
Adv. Synth. Catal. 2013, 355, 2793-2797.

64. Jieming Zhang, Hai-Hong Wu and Junliang Zhang*
Cesium Carbonate Mediated Borylation of Aryl Iodides with Diboron in Methanol.
Eur. J. Org. Chem.2013, 6263-6266.

63. Zuliang Chen, Yuanjing Xiao and Junliang Zhang*
Reaction of Two Differently Functionalized Oxiranes with Nickel Perchlorate: A Modular Entry to Highly Substituted 1,3-Dioxolanes.
Eur. J. Org. Chem.2013, 4748-4751.

62. Renrong Liu and Junliang Zhang*
Organocatalytic Michael Addition of Indoles to Isatylidene-3-acetaldehydes: Application to the Formal Total Synthesis of (-)-Chimonanthine.
Org. Lett. 2013, 15, 2266-2269..

61. Renrong Liu and Junliang Zhang*
Organocatalytic Michael Addition of Malonates to Isatylidene-3-acetaldehydes: Application to the Total Synthesis of (-)-Debromoflustramine E.
Chem. Eur. J. 2013, 19, 7319-7323.

60. Deyun Qian and Junliang Zhang*
Gold/Bronsted Acid Relay Catalysis for Enantioselective Construction of Spirocyclic Diketones.
Chem. Eur. J. 2013, 19, 6984-6988.

2012年
59. Xingxing Wu, Lei Liand Junliang Zhang*
Direct aza-Darzens Aziridination of N-Tosyl Imines with 2-Bromomalonates for Efficient Synthesis of Highly Functionalized Donor-acceptor Aziridines.
Adv. Synth. Catal. 2012, 354, 3485-3489.
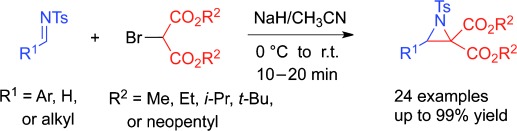
58. Xingxing Wu and Junliang Zhang*
Y(OTf)3-Catalyzed Diastereoselective [3+2]Cycloaddition of N-Tosylaziridines and Imines; Efficient Synthesis of Multisubstituted Imidazolidines.
Synthesis 2012, 44, 2147-2154.

57. Yanqing Zhang and Junliang Zhang*
Highly Substituted Pyrroles by Gold(I)-Catalyzed Tandem Reaction of 1-(1-Alkynyl)cyclopropyl Oxime Ethers with Nucleophiles.
Synlett,2012, 23, 1389-1393.

56. Renrong Liu and Junliang Zhang*
Palladium(II)-Catalyzed Stereospecific Three-Component Domino Reactions of Diyne-enones, Nucleophiles, and Vinyl Ketones.
Chem. Asian. J. 2012. 7, 294-297.

55. Wenbo Li, Yuying Li, Guanghua Zhou, Xiangshu Wu, and Junliang Zhang*
Gold(I)-Catalyzed Regiodivergent Rearrangements: 1,2- and 1,2’-Alkyl Migration in Skipped Alkynyl Ketones.
Chem. Eur. J. 2012, 18, 15113-15121.

54. Xiuzhao Yu, and Junliang Zhang*
A Base-Promoted Tandem Cycloaddition/Air Oxidation Reaction of Electron-Deficient Conjugated Enynes and Hydrazines: Synthesis of Highly Substituted Pyrazoles.
Chem. Eur. J. 2012, 18, 12945-12949.

53. Yanqing Zhang, Junliang Zhang*
Gold(I)-Catalyzed Regio- and Stereoselective 1,3-Dipolar Cycloaddition Reactions of 1-(1-Alkynyl)cyclopropyl Oximes with Nitrones: A Modular Entry to Highly Substituted Pyrrolo[3,4-d][1,2]oxazepines.
Adv. Synth. Catal.2012‚ 354‚ 2556-2560.

52. Zuliang Chen, Ziqi Tian, Jieming Zhang, Jing Ma*, Junliang Zhang*
C-O Versus C-C Bond Cleavage: Selectivity Control in Lewis Acid Catalyzed Chemodivergent Cycloadditions of Aryl Oxiranyldicarboxylates with Aldehydes, and Theoretical Rationalizations of Reaction Pathways.
Chem. Eur. J. 2012,18, 8591-8595.

51. Hongyin Gao, Junliang Zhang*
Cationic Rhodium(I)-Catalyzed Regioselective Tandem Heterocyclization/[3+2] Cycloaddition of 2-(1-Alkynyl)-2-alken-1-ones with Alkynes.
Chem. Eur. J. 2012,18, 2777-2782.

50. Deyun Qian, Junliang Zhang*
Catalytic oxidation/C–H functionalization of N-arylpropiolamides by means of gold carbenoids: concise route to 3-acyloxindoles.
Chem. Commun. 2012,48, 7082-7084.
49. Mei Zhang, Junliang Zhang*
Gold(I)-catalyzed cyclization of 2-(1-alkynyl)-alk-2-en-1-one oximes: a facile access to highly substituted N-alkoxypyrroles.
Chem. Commun. 2012,48, 6399-6401.
48. Xiuzhao Yu, Guanghua Zhou, Junliang Zhang*
DBU-catalyzed tandem additions of electron-deficient 1,3-conjugated enynes with 2-aminomalonates: a facile access to highly substituted 2-pyrrolines.
Chem. Commun. 2012,48, 4002-4004.
47. Yanqing Zhang, Junliang Zhang*
Kinetic resolution of 1-(1-alkynyl)cyclopropyl ketones by gold(I)-catalyzed asymmetric [4+3]cycloaddition with nitrones: scope, mechanism and applications.
Chem. Commun. 2012,48, 4710-4712.
46. Lai Wei, Junliang Zhang*
Efficient synthesis of isochromanones and isoquinolines viaYb(OTf)3-catalyzed tandem oxirane/aziridine ring opening/Friedel–Crafts cyclization.
Chem. Commun. 2012,48, 2636-2638.
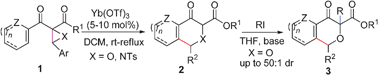
45. Jieming Zhang, Junliang Zhang*
Ni(ClO4)2-catalysed regio- and diastereoselective [3+2] cycloaddition of indoles and aryl oxiranyl-dicarboxylates/diketones: a facile access to furo[3,4-b]indoles.
Chem. Commun. 2012,48, 1817-1819.
44. Jian-Jun Feng‚Junliang Zhang*
J. Am. Chem. Soc. 2011,133‚7304–7307.

43. Deyun Qian, Junliang Zhang*
A gold(I)-catalyzed intramolecular oxidation–cyclopropanation sequence of 1,6-enynes: a convenient access to [n.1.0]bicycloalkanes.
Chem. Commun. 2011,47, 11152-11154.
42. Xingxing Wu, Lei Li, Junliang Zhang*
Nickel(II)-catalyzed diastereoselective [3+2] cycloaddition of N-tosyl-aziridines and aldehydes via selective carbon–carbon bond cleavage.
Chem. Commun. 2011,47, 7824-7826.
![Graphical abstract Graphical abstract:Nickel(ii)-catalyzed diastereoselective [3+2] cycloaddition of N-tosyl-aziridines and aldehydes via selective carbon–carbon bond cleavage](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1CC12189H)
41. Lei Li,Xingxing Wu, Junliang Zhang*
Lewis acid-catalyzed formal [3+2] cycloadditions of N-tosyl aziridines with electron-rich alkenes via selective carbon–carbon bond cleavage.
Chem. Commun. 2011,47, 5049-5051.
40. Lei Li‚ Junliang Zhang*
Lewis Acid-catalyzed [3 + 2]Cyclo-addition of Alkynes with N-Tosyl-aziridines via Carbon–Carbon Bond Cleavage: Synthesis of Highly Substituted 3-Pyrrolines.
Org. Lett.2011‚13‚ 5940-5943.

39. Zuliang Chen‚ Lai Wei‚ Zhang Junliang*
Lewis Acid Catalyzed Carbon-Carbon Bond Cleavage of Aryl Oxiranyl Diketones: Synthesis of cis-2‚5-Disubstituted 1‚3-Dioxolanes.
Org. Lett.2011‚13‚ 1170-1173.

38. Renrong Liu, Mei Zhang‚ Junliang Zhang*
Highly regioselective Lewis acid-catalyzed [3+2] cycloaddition of alkynes with donor–acceptor oxiranes by selective carbon–carbon bond cleavage of epoxides.
Chem. Commun. 2011,47, 12870-12872.
37. Xiuzhao Yu‚ Junliang Zhang*
Adv. Synth. Catal.2011‚ 353‚ 1265-1268.

36. Deyun Qian‚ Junliang Zhang*
Au(I)/Au(III)-catalyzed Sonogashira-type reactions of functionalized terminal alkynes with arylboronic acids under mild conditions.
Beilstein J. Org. Chem. 2011‚ 7‚ 808–812. (Invited contribution in honor of F. Dean Toste )
![Graphical abstract Graphical abstract:A gold(i)-catalyzed intramolecular oxidation–cyclopropanation sequence of 1,6-enynes:a convenient access to [n.1.0]bicycloalkanes](https://faculty.ecnu.edu.cn/picture/article/1811/e3/9f/a1b8fd8f41e8b5aa874a58797541/664ff6b5-eb86-4ca5-a33d-52842c6a5a77.gif.x)
35. Hongyin Gao‚ Xingxing Wu‚ Junliang Zhang*
Gold(I)-Catalyzed‚ Highly Diastereoselective‚ Tandem Heterocyclizations/[3+2] Cycloadditions: Synthesis of Highly Substituted Cyclopenta[c]furans.
Chem. Eur. J. 2011‚ 17‚ 2838-28414.

34. Guanghua Zhou‚ Feng Liu‚ Junliang Zhang*
Enantioselective Gold-Catalyzed Functionalization of Unreactive sp3 C-H Bonds through a Redox–Neutral Domino Reaction.
Chem. Eur. J. 2011‚ 17‚3101-3104.

33. Tao Wang‚ Junliang Zhang*
Chemoselective C-C Bond Cleavage of Epoxide Motifs: Gold(I)-Catalyzed Diastereoselective [4+3] Cycloadditions of 1-(1-Alkynyl)oxiranyl Ketones and Nitrones.
Chem. Eur. J. 2011‚ 17‚ 86-90.

32. Tao Wang‚ Cui-Hong Wang ‚ Junliang Zhang* Unexpected C–C bond cleavage of epoxide motif: Rhodium(i)-catalyzed tandem heterocyclization/[4+1] cycloaddition of 1-(1-alkynyl)oxiranyl ketones.
Chem. Commun. 2011‚ 47‚ 5578-5580.
31. Wanxiang Zhao‚Junliang Zhang*
Rhodium-Catalyzed Tandem Heterocyclization and Carbonylative [(3+2)+1] Cyclization of Diyne-enones.
Org. Lett. 2011‚13‚688–691.

30. Renrong Liu‚ Junliang Zhang*
Polyheterocycles by Palladium(II)-Catalyzed Oxidative Domino Reactions Involving Direct C-H Functionalization.
Adv. Synth. Catal. 2011‚ 353‚ 36-40.

2010年
29. Liu Lu‚ Wei Lai‚ Zhang Junliang*
A Facile Route to Polysubstituted Naphthalenes and Benzofluorenols via Scandium Triflate- and Triflic Acid- Catalyzed Benzannulation of 2-(2-Alkynylarylidene)- 1,3-Dicarbonyl Compounds.
Adv. Synth. Catal. 2010‚352‚1920-1924.

28. Xiuzhao Yu‚ Bo Du‚ Kai Wang and Junliang Zhang*
Highly Substituted 2,3-Dihydroisoxazoles by Et3N-Catalyzed Tandem Reaction of Electron-Deficient 1,3-Conjugated Enynes with Hydroxylamines.
Org. Lett. 2010‚12‚1876–1879.

27. Xiuzhao Yu‚Zhongyan Cao‚Junliang Zhang*
Organocatalytic hetero [4+2] cycloaddition reactions of 2-(1-alkynyl)-2-alkene-1-ones: metal-free access to highly substituted 4H-pyrans.
Org. Biomol. Chem. 2010‚8‚5059-5061.
26. Wanxiang Zhao‚ Junliang Zhang*
Rhodium-catalyzed domino heterocyclization and [(3+2)+2] carbocyclization: construction of fused tricycloheptadienes.
Chem. Commun. 2010‚ 46‚ 7816-7818.
25. Hongyin Gao‚ Xingxing Wu‚ Junliang Zhang*
Exo/endo selectivity-control in Lewis-acid catalyzed tandem heterocyclization/formal [4+3] cycloaddition: synthesis of polyheterocycles from 2-(1-alkynyl)-2-alken-1-ones and 1,3-diphenylisobenzofuran.
Chem. Commun. 2010‚ 46‚ 8764-8766.
24. Wenbo Li‚ Junliang Zhang*
Tetrasubstituted furans by PdII/CuI-cocatalyzed three-component domino reactions of 2-(1-alkynyl)-2-alken-1-ones, nucleophiles and diaryliodonium salts.
Chem. Commun.2010‚ 46‚ 8839-8841.
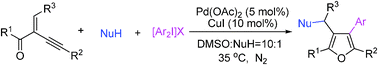
23. Lu Liu‚ Lai Wei‚ Yong Lu‚ Junliang Zhang*
One-Pot Tandem Catalysis: A Concise Route to Fused Bicyclic Scaffolds from Acyclic β-Ketoesters and Alkynyl Aldehydes
Chem. Eur. J. 2010‚ 16‚ 11813-11817.

22. Guanghua Zhou‚Junliang Zhang*
Product-selectivity control by the nature of the catalyst: Lewis acid-catalyzed selective formation of ring-fused tetrahydroquinolines and tetrahydroazepines via intramolecular redox reaction.
Chem. Commun. 2010‚46‚6593–6595.
21. Feng Liu‚Deyun Qian‚Lei Li‚ Xiaoli Zhao‚Junliang Zhang*
Angew. Chem. Int. Ed. 2010‚49‚ 6669-6672.

20. Zuliang Chen‚ Junliang Zhang*
An Unexpected Phosphine-Catalyzed Regio- and Diastereoselective [4+1] Annulation Reaction of Modified Allylic Compounds with Activated Enones.
Chem. Ansia J. 2010‚ 5, 1542-1545.

19. Wenbo Li‚ Yuying Li‚ Junliang Zhang*
Gold-Catalyzed Domino Reactions Consisting of Regio- and Stereoselective 1‚2-Alkyl Migration.
Chem. Eur. J. 2010‚ 16‚ 6447-6450.

18. Yanqing Zhang‚ Feng Liu‚ Junliang Zhang*
Catalytic Regioselective Control in the Diastereoselective 1‚3-Dipolar Cycloaddition Reactions of 1-(1-Alkynyl)cyclopropyl Ketones with Nitrones.
Chem. Eur. J. 2010‚16‚ 6146-6150.

17. Wanxiang Zhao‚ Junliang Zhang*
Rhodium-catalyzed tandem nucleophilic addition/bicyclization of diyne-enones with alcohols: a modular entry to 2‚3-fused bicyclic furans.
Chem. Commun. 2010‚ 46‚ 4384-4386.
16. Tao Wang‚ Junliang Zhang*
Synthesis of 2-acylfurans from 3-(1-alkynyl)-2-alken-1-ones via the oxidation of gold-carbene intermediates by H2O2.
Dalton Trans. 2010‚ 39‚ 4270-4273.
15. Xiuzhao Yu‚ Bo Du‚ Kai Wang‚ Junliang Zhang*
Highly Substituted 2‚3-Dihydroisoxazoles by Et3N-Catalyzed Tandem Reaction of Electron-Deficient 1‚3-Conjugated Enynes with Hydroxylamines.
Org. Lett. 2010‚ 12‚ 1876-1879.

14. Hongyin Gao‚ Xiaoli Zhao‚ Yihua Yu‚ Junliang Zhang*
Highly Substituted Furo[3‚4-c]azepines by Gold(I)-Catalyzed Diastereoselective Tandem Double Heterocyclizations and 1‚2-Alkyl Migration.
Chem. Eur. J. 2010‚ 16‚ 456-459.

13. Yuanjing Xiao‚ Junliang Zhang*
Tetrasubstituted allenes by Pd(0)-catalyzed three-component tandem Michael addition/cross-coupling reaction.
Chem. Commun. 2010‚ 46‚ 752-754.
Selectivity Control in Lewis Acid Catalyzed Regiodivergent Tandem Cationic Cyclization/Ring Expansion Terminated by Pinacol Rearrangement.
Angew. Chem. Int. Ed. 2009‚48‚6093-6096.

11. Feng Liu‚ Yihua Yu‚ and Junliang Zhang*
Angew. Chem. Int. Ed. 2009‚48‚5505–5508.

10. Wenbo Li‚ Yuanjing Xiao‚ Junliang Zhang* Alkynyl Group as Activating Group: Base-Catalyzed Diastereoselective Domino Reactions of Electron-Deficient Enynes.
Adv. Synth. Catal. 2009‚ 351‚ 3083-3088.

9. Zuliang Chen‚ Junliang Zhang*Highly Functionalized 4-Alkylidenebicyclo[3.1.0]hex-2-enes by Tandem Michael Addition and Annulation of Electron-Deficient Enynes.
Chem. Asian J. 2009‚ 4‚ 1527-1529.

8. Renrong Liu‚ Junliang Zhang*Tetrasubstituted Furans by Pd-II-Catalyzed Three-Component Domino Reactions of 2-(1-Alkynyl)-2-alken-1-ones with Nucleophiles and Vinyl Ketones or Acrolein.
Chem. Eur. J. 2009‚ 15‚ 9303-9306.

7. Yuanjing Xiao‚ Junliang Zhang*
Furans versus 4H-pyrans: catalyst-controlled regiodivergent tandem Michael addition–cyclization reaction of 2-(1-alkynyl)-2-alken-1-ones with 1‚3-dicarbonyl compounds.
Chem. Commun. 2009‚ 3594-3596.
6. Yanqing Zhang‚ Zuliang Chen‚ Yuanjing Xiao‚ Junliang Zhang*
RhI-Catalyzed Regio- and Stereospecific Carbonylation of 1-(1-Alkynyl)cyclopropyl Ketones: A Modular Entry to Highly Substituted 5‚6-Dihydrocyclopenta[c]furan-4-ones.
Chem. Eur. J. 2009‚ 15‚ 5208-5211.

5. Yuanjing Xiao‚ Junliang Zhang*
Palladium(II)-Catalyzed Domino Reaction of 2-(1-Alkynyl)-2-alken-1-ones with Nucleophiles: Scope‚ Mechanism and Synthetic Application in the Synthesis of 3‚4-Fused Bicyclic Tetrasubstituted Furans.
Adv. Synth. Catal. 2009‚ 351‚ 617.

4. Hongyin Gao‚ Junliang Zhang*
A Dramatic Substituent Effect in Silver(I)-Catalyzed Regioselective Cyclization of ortho-Alkynylaryl Aldehyde Oxime Derivatives.
Adv. Synth. Catal. 2009‚ 351‚ 85.

2008年
3. Liyan Fan‚ Wanxiang Zhao‚ Weihua Jiang‚ Junliang Zhang*
One-Pot Synthesis of Fused Tricyclic Heterocycles with Quaternary Carbon Stereocenter by Sequential Pauson–Khand Reaction and Formal [3+3] Cycloaddition.
Chem. Eur. J. 2008‚14‚ 9139-9142.

2. Xiuzhao Yu‚ Hongjun Ren‚ Yuanjing Xiao‚ Junliang Zhang*. Efficient Assembly of Allenes, 1,3-Dienes, and 4H-Pyrans by Catalytic Regioselective Nucleophilic Addition to Electron-Deficient 1,3-Conjugated Enynes.
Chem. Eur. J. 2008‚ 14‚ 8481-8485.

1. Yuanjing Xiao and Junliang Zhang*
Angew. Chem. Int. Ed. 2008‚47‚1903-1906.










![Graphical abstract: Gold-catalyzed intermolecular [4+1] spiroannulation via site-selective aromatic C(sp2)–H functionalization and dearomatization of phenol derivatives](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0CC01118E&imageInfo.ImageIdentifier.Year=2020)
![Graphical abstract: Enantiodivergent synthesis of 1,2-bis(diphenylphosphino)ethanes via asymmetric [3 + 2]-cycloaddition](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8QO01404C&imageInfo.ImageIdentifier.Year=2019)

![Graphical abstract: Enantioselective [3+2] cycloaddition of azomethine ylides and aldehydes via Ni/bis(oxazoline)-catalyzed ring opening of N-tosylaziridines through a chirality transfer approach](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CC02906C&imageInfo.ImageIdentifier.Year=2017)
![Graphical abstract: Rhodium-catalyzed intermolecular [3+3] cycloaddition of vinyl aziridines with C,N-cyclic azomethine imines: stereospecific synthesis of chiral fused tricyclic 1,2,4-hexahydrotriazines](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CC02078C&imageInfo.ImageIdentifier.Year=2017)
![Graphical abstract: Phosphine-catalyzed enantioselective [3 + 2] cycloadditions of γ-substituted allenoates with β-perfluoroalkyl enones](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7SC01432E&imageInfo.ImageIdentifier.Year=2017)
![Graphical abstract: Copper(i)-catalyzed asymmetric exo-selective [3+2] cycloaddition of azomethine ylides with β-trifluoromethyl β,β-disubstituted enones](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7CC03015K&imageInfo.ImageIdentifier.Year=2017)

![Graphical abstract: Enantioselective gold-catalyzed intermolecular [2 + 2]-cycloadditions of 3-styrylindoles with N-allenyl oxazolidinone](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00087H&imageInfo.ImageIdentifier.Year=2016)

![Graphical abstract: Gold(i)-catalyzed asymmetric [3 + 2]-cycloadditions of γ-1-ethoxyethoxy-propiolates and aldehydes](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4QO00261J&imageInfo.ImageIdentifier.Year=2015)
![Graphical abstract: Scandium-catalyzed tandem selective oxirane ring-opening/Friedel–Crafts alkylation: a facile access to [1,4]oxazino[4,3-a]indoles and 3,4-dihydro-1H-pyrrolo[2,1-c][1,4]oxazines](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C4OB01057D&imageInfo.ImageIdentifier.Year=2014)

![Graphical abstract Graphical abstract:Kinetic resolution of 1-(1-alkynyl)cyclopropyl ketones by gold(i)-catalyzed asymmetric [4+3]cycloaddition with nitrones:scope, mechanism and applications](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2CC30309D)
![Graphical abstract Graphical abstract:Ni(ClO4)2-catalysed regio- and diastereoselective [3+2] cycloaddition of indoles and aryl oxiranyl-dicarboxylates/diketones:a facile access to furo[3,4-b]indoles](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2CC16918E)
![Graphical abstract Graphical abstract:A gold(i)-catalyzed intramolecular oxidation–cyclopropanation sequence of 1,6-enynes:a convenient access to [n.1.0]bicycloalkanes](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1CC14788A)
![Graphical abstract Graphical abstract:Lewis acid-catalyzed formal [3+2] cycloadditions of N-tosyl aziridines with electron-rich alkenes via selective carbon–carbon bond cleavage](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1CC10926J)
![Graphical abstract Graphical abstract:Highly regioselective Lewis acid-catalyzed [3+2] cycloaddition of alkynes with donor–acceptor oxiranes by selective carbon–carbon bond cleavage of epoxides](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1CC15669A)
![Graphical abstract: Unexpected C–C bond cleavage of epoxide motif: Rhodium(i)-catalyzed tandem heterocyclization/[4+1] cycloaddition of 1-(1-alkynyl)oxiranyl ketones](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C0CC05650B&imageInfo.ImageIdentifier.Year=2011)
![Graphical abstract Graphical abstract:Organocatalytic hetero [4+2] cycloaddition reactions of 2-(1-alkynyl)-2-alkene-1-ones:metal-free access to highly substituted 4H-pyrans](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C0OB00334D)
![Graphical abstract Graphical abstract:Rhodium-catalyzed domino heterocyclization and [(3+2)+2] carbocyclization:construction of fused tricycloheptadienes](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C0CC02382E)
![Graphical abstract Graphical abstract:Exo/endo selectivity-control in Lewis-acid catalyzed tandem heterocyclization/formal [4+3] cycloaddition:synthesis of polyheterocycles from 2-(1-alkynyl)-2-alken-1-ones and 1,3-diphenylisobenzofuran](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C0CC02778B)