Independent
Li, Z.-Y.; Huang, C.-D.; Guan, C.-Y.; Guo, H.-M.; Liu, L.-M.; Xiao, X.; Gao, B.*; Ni, S.*; Mei, G.-J.* Cu-Catalyzed Enantioconvergent Oxygen-Centered Radical Cyclization. Chem. Sci. 2025, ASAP. DOI: 10.1039/D5SC07101A.

45. Wu, M.-Y.; Huang, X.-Y.; Gao, B.*; Mei, G.-J.* Organocatalytic Asymmetric Oxa‐[3+3]‐annulation for Chiral Tricyclic Chromans with Contiguous Stereocenters. Chin. J. Chem. 2026. 44, 47-54.. DOI: 10.1002/cjoc.70309.
44. Guan, C.-Y.; Lu, T.; Li, Y.; Liu, C.-H.; Xiao, X.; Mei, G.-J.* Catalytic Enantioselective Construction of Two N-Stereogenic Centers of ethano-and propano-Tröger's Bases. Chem. Sci. 2025, 16, 19683-19693. DOI: 10.1039/D5SC05846E.

43. Li, Y.-W.#; Mo, N.-N.#; Zhang, H.#; Wu, J.-X.; Han, T.-J.; Xiao, X.; Wei, D.*; Mei, G.-J.* Organocatalytic asymmetric synthesis of Tröger's bases. Nat. Commun. 2025, 16, 6383. DOI: 10.1038/s41467-025-61772-4.

42. Yan, X.-B.; Zhao, R.; Miao, Y.-H.; Liu, M.-M.*; Mei, G.-J.* Regioselective N-arylation of N-Acylsulfenamides Enabled by o-Quinone Diimides. Org. Lett. 2025, 27, 2146-2150. DOI: 10.1021/acs.orglett.5c00198.

41. Li, L.#; Li, Y.-Y.#; Li, B.-B.; Shi, L.; Gao, B.*; Mei, G.-J.* Access to (Bridged) Bicyclic Ureas through Azocarboxamide-Enabled Enantioselective 1,2-Diamination of α-Branched Cyclic Ketones. Org. Chem. Front. 2025, 12, 1772-1777. DOI: 10.1039/D4QO02318H. (Invited Paper)

40. Han, T.-J.; Ke, X.-Y.; Wang, M.-C.; Ni, S.-F.*; Mei, G.-J.* A Chemically Powered Rotary Molecular Motor Based on Reversible Oxazepine Formation. Angew. Chem. Int. Ed. 2025, 64, e202418933. DOI: 10.1002/anie.202418933.

39. Fu, Y.-D.#; Zhang, H.#; Li, B.-B.#; Huang, L.*; Xiao, X.; Wang, M.-C.; Wei, D.*; Mei, G.-J.* Azocarboxamide-enabled enantioselective regiodivergnet unsymmetrical 1,2-diaminations. Nat. Commun. 2024, 15, 10225. DOI: 10.1038/s41467-024-54598-z.

38. Han, T.-J.; Yang, Q.-L.; Hu, J.; Wang, M.-C.; Mei, G.-J.* Divergent Synthesis of Chiroptical Molecular Switches Based on 1,2-Diaxial Atropisomers. JACS Au 2024, 4, 4445-4454. DOI: 10.1021/jacsau.4c00777.

37. Gao, X.; Li, B.-B.; Li, Y.-W.; Xiao, X.; Liu, M.-M.*; Mei, G.-J.* Enantiodivergent Cyclization of Racemic Cyclohexadienones via Parallel Kinetic Asymmetric Transformation. Org. Lett. 2024, 26 , 6290-6294. DOI: 10.1021/acs.orglett.4c02396.

36. Huang, X.-Y.#; Li, N.#; Miao, Y.-H.#; Hua, Y.-Z.*; Wang, M.; Xu, L.-P.*; Mei, G.-J.* Reversal of Enantioselectivity by Tuning the Ring Size of ProPhenol. Org. Chem. Front. 2024, 11, 4109-4118. DOI: 10.1039/d4qo00668b.

35. Guan, C.-Y.; Zou, S.; Luo, C.; Li, Z.-Y.; Huang, M.; Huang, L.*; Xiao, X.; Wei, D.; Wang, M.-C.; Mei, G.-J.* Catalytic asymmetric synthesis of planar-chiral dianthranilides via (Dynamic) kinetic resolution. Nat. Commun. 2024, 15 , 4580. DOI: 10.1038/s41467-024-48947-1.

34. Dong, R.; Han, T.-J.; Huang, L.*; Mei, G.-J.* Chemodivergent (4 + 3) Cycloadditions of 2-Indolylmethanols with 1,3,5-Triazinanes: Access to Polycyclic Indoles. Org. Chem. Front. 2024. 11, 3624-3629. DOI: 10.1039/d4qo00657g.

33. Li, Y.-Y.#; Yang, F.-Y.#; Wu, M.-Y.; Huang, L.*; Mei, G.-J.* Catalytic Asymmetric Dearomative Arylation of 2-Naphthols Enabled by o-Quinone Diimides. Adv. Synth. Catal. 2024, 366, 4238-4243. DOI: 10.1002/adsc.202400200. (Invited Paper)

32. Zhang, J.; Sun, W.-N.; Jiang, Z.-W.; Jia, S.-K.*; Mei, G.-J.* Diastereodivergent and Regioselective Synthesis of Tetrahydrofuro[2,3-b]furans with Four Consecutive Stereocenters. J. Org. Chem. 2024. 89, 4134-4144. DOI: 10.1021/acs.joc.4c00069.
31. Luo, C.; Guan, C.-Y.; Li, Z.-Y.; Gao, B.*; Mei, G.-J.* Dearomative Spiroannulation of Indoles Enabled by Diaza-[1,2]-Wittig Rearrangement. Org. Chem. Front. 2024. 11, 1685-1691. DOI: 10.1039/D3QO02033A.

30. Sun, W.-N.; Mei, G.-J.*; Jia, S.-K.* Substrate Directed Regio‐ and Enantioselective Ring‐Opening of Epoxides and Aziridines. ChemCatChem 2024. 16, e202301431. DOI: 10.1002/cctc.202301431.
29. Shi, L.; Li, T.; Zhang, W.*; Hu, W.; Zhu, X.*; Lu, Y.; Mei, G.-J.* Visible-light induced photocatalyst-free difluoromethylation of quinoxalinones with difluorosulfones. Green Synth. Catal. 2024. 5, 277-281. DOI: 10.1016/j.gresc.2023.08.002.

28. Miao, Y.-H.; Zhang, Z.-X.; Huang, X.-Y.; Hua, Y.-Z.; Jia, S.-K.; Xiao, X.; Wang, M.-C.*; Xu, L.-P.*; Mei, G.-J.* Catalytic asymmetric dearomative azo-Diels–Alder reaction of 2-vinlyindoles. Chin. Chem. Lett. 2024, 35, 108830. DOI: 10.1016/j.cclet.2023.108830.

27. Gao, H.-J.#; Miao, Y.-H.#; Sun, W.-N.; Zhao, R.; Xiao, X.; Hua, Y.-Z.; Jia, S.-K.; Wang, M.-C.; Mei, G.-J.* Diversity-Oriented Catalytic Asymmetric Dearomatization of Indoles with o-Quinone Diimides. Adv. Sci. 2023, 10, 2305101. DOI: 10.1002/advs.202305101.

26. Han, T.-J.#; Guan, C.-Y.#; Li, N.; Dong, R.; Xu, L.-P.; Xiao, X.; Wang, M.-C.*; Mei, G.-J.* Catalytic Atroposelective Synthesis of Heterobiaryls with Vicinal C−C and N−N Diaxes via Dynamic Kinetic Resolution. iScience 2023, 26, 107978. DOI: 10.1016/j.isci.2023.107978.

25. Gao, X.; Han, T.-J.; Li, B.-B.; Hou, X.-X.; Hua, Y.-Z.; Jia, S.-K.; Xiao, X.; Wang, M.-C.; Wei, D.; Mei, G.-J.* Catalytic asymmetric dearomatization of phenols via divergent intermolecular (3 + 2) and alkylation reactions. Nat. Commun. 2023, 14, 5189. DOI: 10.1038/s41467-023-40891-w.

24. Jia, S.-K.; Mei, G.-J.* Construction of Axially Chiral Arylpyrroles via Atroposelective Diyne Cyclization. Chin. J. Org. Chem. 2023, 43, 2261-2263. (Invited Highlight)
23. Du, S.-S.; Zhai, Y.-H.; Zhang, C.; Wang, M.-C.*; Jia, S.-K.; Mei, G.-J.*; Hua, Y. Z.* Dinuclear Zinc‐Catalyzed Asymmetric Desymmetrization of Cyclopentendiones: Access to Functional Cyclopentanediones Bearing an All‐carbon Quaternary Stereocenter. Chem. Asian J. 2023, 18, e202300591.
22. Chen, Y.; Jia, S.-K.; Xiao, X.; Wang, M.-C.; Huang, L.*; Mei, G.-J.* Catalytic Asymmetric Synthesis of Aza-Quaternary Carbon Cyclohexadieneones Enabled by Aminative Dearomatization of Phenols. Org. Lett. 2023, 25, 4740-4744. DOI: 10.1021/acs.orglett.3c01746.

21. Fu, Y.-D.; Gao, X.; Jia, S.-K.; Xiao, X.; Wang, M.-C.; Huang, L.*; Mei, G.-J.* Catalyst-free racemic and H2O/CPA-catalyzed asymmetric regio-reversed domino processes of triketone enones with azlactones. Green Chem. 2023, 25, 5692-5697. DOI: 10.1039/D3GC01445B.

20. Chen, Y.-X.; Han, T.-J.; Xiao, X.; Wang, M.-C.*; Mei, G.-J.* Catalytic asymmetric interrupted Attanasi reaction: access to fused 2,3-dihydropyrroles with vicinal quaternary carbons. Chem. Commun. 2023, 59, 8103-8106. DOI: 10.1039/D3CC02172F.

19. Miao, Y.-H.; Hua, Y.-Z.*; Jia, S.-K.; Xiao, X.; Wang, M.-C.*; Mei, G.-J.* Zn-ProPhenol catalyzed asymmetric inverse-electron-demand Diels–Alder reaction. Chem. Commun. 2023, 59, 6929-6932. DOI: 10.1039/d3cc01641b.

18. Mo, N.-N.; Miao, Y.-H.; Xiao, X.; Hua, Y.-Z.; Wang, M.-C.; Huang, L.*; Mei, G.-J.* Catalytic asymmetric de novo construction of 4-pyrrolin-2-ones via intermolecular formal [3 + 2] cycloaddition of azoalkenes with azlactones. Chem. Commun. 2023. 59, 5902-5905. DOI: 10.1039/D3CC01194A.

17. Xu, Z.-H.; Li, N.; Chang, Z.-R.; Hua, Y.-Z.; Xu, L.-P.*; Jia, S.-K.*; Wang, M.-C.*; Mei, G.-J.* Acyl transfer-enabled catalytic asymmetric Michael addition of α-hydroxy-1-indanones to nitroolefins. Chem. Synth. 2023, 3, 17. (invited paper)

16. Yang, F,-Y.; Han, T.-J.; Jia, S.-K.; Wang, M.-C.; Mei, G.-J.* Catalytic [2,3]-sigmatropic rearrangement of sulfonium ylides derived from azoalkenes: non-carbenoid Doyle−Kirmse reaction. Chem. Commun., 2023, 59, 3107-3110. DOI: 10.1039/D3CC00160A.

15. Song, T.-Y.; Li, R.; Huang, L.*; Jia, S.-K.*; Mei, G.-J.* Catalytic Asymmetric Synthesis of N-N Atropisomers. Chin. J. Org. Chem. 2023, 43, 1977-1990. DOI: 10.6023/cjoc202212003. (Invited Review, cover paper)


14. Gao, H.-J.; Miao, Y.-H.; Jia, S.-K.; Li, N.; Xu, L.-P.; Wang, W.; Wang, M.-C.*; Mei, G.-J.* Azo group-enabled metal- and oxidant-free alkenyl C–H thiolation: Access to stereodefined tetrasubstituted acyclic olefins. Green Synth. Catal. 2023, 4, 67-70. DOI: 10.1016/j.gresc.2022.09.001.

13. Guan, C.-Y.; Han, T.-J.; Jia, S.-K.; Hua, Y.-Z.; Mei, G.-J.* Diastereodivergent formal [4 + 1] cycloaddition of azoalkenes as one-carbon synthons. Green Synth. Catal. 2023, 4, 258-262. DOI: 10.1016/j.gresc.2022.05.003.

12. A book chapter: Mei, G.-J.*; Chen, Y.-X.; Shi, F.* Miscellaneous (3 + 2) Cycloadditions. 2022. DOI: 10.1016/b978-0-32-390644-9.00023-8. (invited paper)
11. Shi, L.*; An, D.; Mei, G.-J.* Difluoromethylation of Heterocycles via a Radical Process. Org. Chem. Front. 2022. 9, 4192–4208. DOI: 10.1039/d2qo00762b. (invited paper)

10. Han, T.-J.#; Zhang, Z.-X.#; Wang, M.-C.*; Xu, L.-P.*; Mei, G.-J.* The Rational Design and Atroposelective Synthesis of Axially Chiral C2‐Arylpyrrole‐Derived Amino Alcohols. Angew. Chem. Int. Ed. 2022. 61, e202207517. DOI: 10.1002/anie.202207517.

9. Miao, Y.-H.; Hua, Y.-Z.*; Gao, H.-J.; Mo, N.-N.; Wang, M.-C.*; Mei, G.-J.* Catalytic asymmetric inverse-electron-demand aza-Diels–Alder reaction of 1,3-diazadienes with 3-vinylindoles. Chem. Commun. 2022. 58, 7515–7518. DOI: 10.1039/D2CC02458F.

8. Shi, L.#*; Li, T.#; Mei, G.-J.* Recent advances in transition-metal-free C–H functionalization of imidazo[1,2-a]pyridines. Green Synth. Catal. 2022, 3, 227–242. DOI: 10.1016/j.gresc.2022.03.007.

7. Mei, G.-J.*; Koay, W. L.; Guan, C.-Y.; Lu, Y.* Atropisomers beyond the C–C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2022, 8, 1855–1893. DOI: 10.1016/j.chempr.2022.1004.1011.
6. Xu, Z.-H.; Jia, S.-K.*; Chang, Z.-R.; Hua, Y.-Z.; Wang, M.-C.*; Mei, G.-J.* Facile Access to Saccharin‐Fused 1,4‐Dihydropyridines through [3+3] Annulation Reactions. Eur. J. Org. Chem. 2022, 2022, e202101423. (Invited Paper)
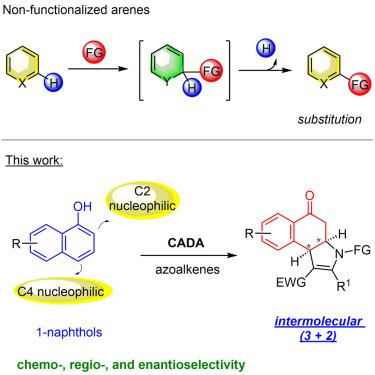
5. Mei, G.-J.#*; Luo, Y.#; Koay, W. L.; Li, R.; Lan, Y.*; Lu, Y.* Chemo-, regio- andenantioselective dearomative (3+2) reaction of non-functionalized 1-naphthols. Chem Catal. 2022, 2, 386-399.
4. Deng, R.*; Han, T.-J.; Gao, X.; Yang, Y.-F.; Mei, G.-J.* Further developments of β,γ-unsaturated α-ketoesters as versatile synthons in asymmetric catalysis. iScience 2022, 25, 103913.

3. Han, T.-J.; Wang, M.-C.*; Mei, G.-J.* 2-Indolymethanols as 4-atom-synthons in oxa-Michael reaction cascade: access to tetracyclic indoles. Chem. Commun. 2021, 57, 8921-8924.
2. Mei, G.-J.; Wong, J. J.; Zheng, W.; Nangia, A. A.; Houk, K. N.; Lu, Y.* Rational design and atroposelective synthesis of N–N axially chiral compounds. Chem 2021, 7, 2743–2757.

1. Mei, G.-J.; Koay, W. L.; Tan, C. X. A.; Lu, Y.* Catalytic asymmetric preparation of pyrroloindolines: strategies and applications to total synthesis. Chem. Soc. Rev. 2021, 50, 5985-6012.
Before ZZU
1. Koay, W. L.; Mei, G.-J.*; Lu, Y.* Facile access to benzofuran-fused tetrahydropyridines via catalytic asymmetric [4 + 2] cycloaddition of aurone-derived 1-azadienes with 3-vinylindoles. Org. Chem. Front. 2021, 8, 968-974;
2. Alvin Tan, C. X.; Mei, G.-J.*; Lu, Y.* Phosphine-Catalyzed Asymmetric Allylic Alkylation of Achiral MBH Carbonates with 3,3'-Bisindolines: Enantioselective Construction of Quaternary Stereogenic Centers. Org. Lett. 2021, 23, 1787-1792;
3. Tasdan, Y.; Mei, G.-J.*; Lu, Y.* Enantioselective synthesis of mixed 3,3′-bisindoles via a phosphine-catalyzed umpolung γ-addition of 3′-indolyl-3-oxindoles to allenoates. Sci. Bull. 2020, 65, 557-563;
4. Mei, G.-J.; Zheng, W.; Gonçalves, T. P.; Tang, X.; Huang, K.-W.; Lu, Y.* Catalytic Asymmetric Formal [3+2] Cycloaddition of Azoalkenes with 3-Vinylindoles: Synthesis of 2,3-Dihydropyrroles. iScience 2020, 23, 100873;
5. Mei, G.-J.; Tang, X.; Tasdan, Y.; Lu, Y.* Enantioselective Dearomatization of Indoles by an Azoalkene-Enabled (3+2) Reaction: Access to Pyrroloindolines. Angew. Chem. Int. Ed. 2020, 59, 648-652;
6. Hang, Q.-Q.; Liu, S.-J.; Yu, L.; Sun, T.-T.; Zhang, Y.-C.*; Mei, G.-J.*; Shi, F.* Design and Application of Indole‐Based Allylic Donors for Pd‐Catalyzed Decarboxylative Allylation Reactions. Chin. J. Chem. 2020, 38, 1612-1618;
7. Yu, L.; Zhu, Z.-Q.; Sun, M.; Mei, G.-J.*; Shi, F.* C3-Allylation of Indoles via an Iridium-Catalyzed Branch-Selective Ring-Opening Reaction of Vinylcyclopropanes. Synthesis 2019, 51, 1655-1661;
8. Wang, J.-R.; Jiang, X.-L.; Hang, Q.-Q.; Zhang, S.; Mei, G.-J.*; Shi, F.* Catalytic Asymmetric Conjugate Addition of Indoles to para-Quinone Methide Derivatives. J. Org. Chem. 2019, 84, 7829-7839;
9. Jin, L.-W.; Jiang, F.; Chen, K.-W.; Du, B.-X.*; Mei, G.-J.*; Shi, F.* Phosphine-catalyzed regiospecific (3 + 2) cyclization of 3-nitroindoles with allene esters. Org. Biomol. Chem. 2019, 17, 3894-3901;
10. Chan, W. L.; Tang, X.; Zhang, F.; Quek, G.; Mei, G.-J.*; Lu, Y.* Phosphine-Catalyzed (3+2) Annulation of Isoindigos with Allenes: Enantioselective Formation of Two Vicinal Quaternary Stereogenic Centers. Angew. Chem. Int. Ed. 2019, 58, 6260-6264;
11. Zhu, Z.-Q.; Yu, L.; Sun, M.; Mei, G.-J.*; Shi, F.* Regioselective [3+3] Cyclization of 2-Indolymethanols with Vinylcyclopropanes via Metal Catalysis. Adv. Synth. Catal. 2018, 360, 3109-3116;
12. Wu, P.; Wu, J.-L.; Wang, J.-Y.; Mei, G.-J.* Catalytic Asymmetric Dehydrative Arylation of 3-Indolylmethanols with Tryptophols: Enantioselective Synthesis of Bisindolyl-Substituted Triarylmethanes. Chin. J. Org. Chem. 2018, 38, 1251;
13. Wang, J.-Y.; Wu, P.; Wu, J.-L.; Mei, G.-J.*; Shi, F.* Chemodivergent Tandem Cyclizations of 2-Indolylmethanols with Tryptophols: C-N versus C-C Bond Formation. J. Org. Chem. 2018, 83, 5931-5946;
14. Wang, H.-Q.; Xu, M.-M.; Wan, Y.; Mao, Y.-J.; Mei, G.-J.*; Shi, F.* Application of 7-Indolylmethanols in Catalytic Asymmetric Arylations with Tryptamines: Enantioselective Synthesis of 7-indolylmethanes. Adv. Synth. Catal. 2018, 360, 1850-1860;
15. Wan, Y.; Wang, H.-Q.; Xu, M.-M.; Mei, G.-J.*; Shi, F.* Direct C3-arylations of 2-indolylmethanols with tryptamines and tryptophols via an umpolung strategy. Org. Biomol. Chem. 2018, 16, 1536-1542;
16. Mei, G.-J.*; Xu, S.-L.; Zheng, W.-Q.; Bian, C.-Y.; Shi, F.* [4+2] Cyclization of para-Quinone Methide Derivatives with Alkynes. J. Org. Chem. 2018, 83, 1414-1421;
17. Mei, G.-J.; Shi, F.* Catalytic asymmetric synthesis of spirooxindoles: recent developments. Chem. Commun. 2018, 54, 6607-6621;
18. Ma, C.; Zhou, J.-Y.; Zhang, Y.-Z.; Mei, G.-J.*; Shi, F.* Catalytic Asymmetric [2+3] Cyclizations of Azlactones with Azonaphthalenes. Angew. Chem. Int. Ed. 2018, 57, 5398-5402;
19. Lu, Y.-N.; Lan, J.-P.; Mao, Y.-J.; Wang, Y.-X.; Mei, G.-J.*; Shi, F.* Catalytic asymmetric de novo construction of dihydroquinazolinone scaffolds via enantioselective decarboxylative [4+2] cycloadditions. Chem. Commun. 2018, 54, 13527-13530;
20. Liu, S.-J.; Jiang, X.-L.; Wu, S.-F.; Tu, M.-S.*; Mei, G.-J.*; Shi, F.* Efficient Synthesis of Chromenes from Vinyl o-Quinone Methides via a Brønsted Acid Catalyzed Electrocyclization Process. Synthesis 2018, 50, 2416-2422;
21. Liu, J.-X.; Zhu, Z.-Q.; Yu, L.; Du, B.-X.; Mei, G.-J.*; Shi, F.* Bronsted Acid Catalyzed Dehydrative Arylation of 4-Indolylmethanols with Indoles: Efficient Access to Indolyl-Substituted Triarylmethanes. Synthesis 2018, 50, 3436-3444;
22. Li, L.-Z.; Wang, C.-S.; Guo, W.-F.; Mei, G.-J.*; Shi, F.* Catalytic Asymmetric [4+2] Cycloaddition of in Situ Generated o-Quinone Methide (mines with o-Hydroxystyrenes: Diastereo- and Enantioselective Construction of Tetrahydroquinoline Frameworks. J. Org. Chem. 2018, 83, 614-623;
23. Jiang, X.-L.; Wu, S.-F.; Wang, J.-R.; Lu, H.; Mei, G.-J.*; Shi, F.* The [4 + 2] cyclization/retro-Mannich reaction cascade of para-quinone methide derivatives with Pd-containing 1,4-dipoles. Org. Biomol. Chem. 2018, 16, 8395-8402;
24. Jiang, F.; Yuan, F.-R.; Jin, L.-W.; Mei, G.-J.*; Shi, F.* Metal-Catalyzed (4 + 3) Cyclization of Vinyl Aziridines with para-Quinone Methide Derivatives. ACS Catal. 2018, 8, 10234-10240;
25. Cao, Z.; Zhou, G.-X.; Ma, C.; Jiang, K.; Mei, G.-J.* Brønsted Acid Catalyzed Domino 1,6-Addition/Intramolecular Cyclization Reactions: Diastereoselective Synthesis of Dihydrocoumarin Frameworks. Synthesis 2018, 50, 1307-1314;
26. Bian, C.-Y.; Li, D.; Shi, Q.; Mei, G.-J.*; Shi, F.* Brønsted Acid Catalyzed Dehydrative Nucleophilic Substitution of C3-Substituted 2-Indolylmethanols with Azlactones. Synthesis 2018, 50, 295-302;
27. Zhu, Z.-Q.; Yin, L.; Wang, Y.; Shen, Y.; Li, C.; Mei, G.-J.*; Shi, F.* Diastereo- and enantioselective construction of biologically important pyrrolo[1,2-a]indole scaffolds via catalytic asymmetric [3+2] cyclodimerizations of 3-alkyl-2-vinylindoles. Org. Chem. Front. 2017, 4, 57-68;
28. Shen, Y.; Zhu, Z.-Q.; Liu, J.-X.; Yu, L.; Du, B.-X.*; Mei, G.-J.*; Shi, F.* BrOnsted Acid Catalyzed C3-Alkylation of 2-Indolylmethanols with Azlactones via an Umpolung Strategy. Synthesis 2017, 49, 4025-4034;
29. Mei, G.-J.#; Zhu, Z.-Q.#; Zhao, J.-J.; Bian, C.-Y.; Chen, J.; Chen, R.-W.; Shi, F.* Bronsted acid-catalyzed stereoselective [4+3] cycloadditions of ortho-hydroxybenzyl alcohols with N,N'-cyclic azomethine imines. Chem. Commun. 2017, 53, 2768-2771;
30. Mei, G.-J.; Shi, F.* Indolylmethanols as Reactants in Catalytic Asymmetric Reactions. J. Org. Chem. 2017, 82, 7695-7707;
31. Mei, G.-J.*; Li, D.; Zhou, G. X.; Shi, Q.; Cao, Z.; Shi, F.* A catalytic asymmetric construction of a tetrahydroquinoline-based spirooxindole framework via a diastereo- and enantioselective decarboxylative [4+2] cycloaddition. Chem. Commun. 2017, 53, 10030-10033;
32. Mei, G.-J.*; Bian, C.-Y.; Li, G.-H.; Xu, S.-L.; Zheng, W.-Q.; Shi, F.* Catalytic Asymmetric Construction of the Tryptanthrin Skeleton via an Enantioselective Decarboxylative [4 + 2] Cyclization. Org. Lett. 2017, 19, 3219-3222;
33. Li, C.; Lu, H.; Sun, X.-X.; Mei, G.-J.*; Shi, F.* Diastereo- and enantioselective construction of spirooxindole scaffolds through a catalytic asymmetric [3 + 3] cycloaddition. Org. Biomol. Chem. 2017, 15, 4794-4797;
34. Jiang, X.-L.; Liu, S.-J.; Gu, Y.-Q.; Mei, G.-J.*; Shi, F.* Catalytic Asymmetric [4+1] Cyclization of ortho-Quinone Methides with 3-Chlorooxindoles. Adv. Synth. Catal. 2017, 359, 3341-3346;
35. He, Y.-Y.; Sun, X.-X.; Li, G.-H.; Mei, G.-J.*; Shi, F.* Substrate-Controlled Regioselective Arylations of 2-Indolylmethanols with Indoles: Synthesis of Bis(indolyl)methane and 3,3'-Bisindole Derivatives. J. Org. Chem. 2017, 82, 2462-2471;
36. Wang, C.-S.; Fan, T.; Zhang, H.-H.; Li, C.; Shen, Y.; Mei, G.-J.*; Shi, F.* Gallium Bromide-Promoted Dearomative Indole Insertion in 3-Indolylmethanols: Chemoselective and (Z/E)-Selective Synthesis of 3,3'-Bisindole Derivatives. J. Org. Chem. 2016, 81, 11734-11742;
37. Mei, G.-J.; Shi, F.* Design and Application of 3-Alkyl-2-vinylindoles in BrOnsted Acid Catalyzed Reactions. Synlett 2016, 27, 2515-2524;
38. Mei, G.-J.; Liu, X.; Qiao, C.; Chen, W.; Li, C. C.* Type II Intramolecular [5+2] Cycloaddition: Facile Synthesis of Highly Functionalized Bridged Ring Systems. Angew. Chem. Int. Ed. 2015, 54, 1754-1758;
39. Mei, G.-J.; Yuan, H.; Gu, Y.-Q.; Chen, W.; Chung, L. W.; Li, C. C.* Dearomative Indole [5+2] Cycloaddition Reactions: Stereoselective Synthesis of Highly Functionalized Cyclohepta[b]indoles. Angew. Chem. Int. Ed. 2014, 53, 11051-11055;
40. Liu, G.#; Mei, G.-J.#; Chen, R. W.; Yuan, H. N.; Yang, Z.; Li, C. C.* Total Synthesis of Aplykurodinone-1. Org. Lett. 2014, 16, 4380-4383;
41. Mei, G.-J.; Li, Z.-F.; Zhang, H.-F.; Li, G.*; Meng, X.-R. Synthesis, Structures, and Properties of Two Novel Zn(II) Polynuclear Complexes. Synth. React. Inorg. M. 2010, 40, 163-169.