Abstract
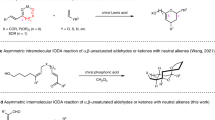
Compared with the conventional Diels–Alder reaction, the development of selective cross-Diels–Alder reactions between two different conjugated dienes, especially in a catalytic asymmetric manner, has been neglected. We now report a peri- and enantioselective cross-Diels–Alder reaction of 3-alkoxycarbonyl-2-pyrones with unactivated conjugated dienes catalysed by a copper(II)–bis(oxazoline) complex, leading to formal inverse-electron-demand adducts with high enantioselectivity under mild reaction conditions. Computational studies showed that this reaction proceeds through an ambimodal transition state: post-transition-state bifurcation leads to [2+4] and [4+2] adducts with the same enantioselectivity, followed by a facile Cope rearrangement to provide a single observed thermodynamic [2+4] product. This reaction occurs with a wide variety of cyclopentadienes, fulvenes and cyclohexadienes, providing a highly efficient and enantioselective approach to densely functionalized cis-bicyclic scaffolds. The synthetic value of this reaction is demonstrated by the asymmetric synthesis of two biologically important natural products, artemisinic acid and coronafacic acid.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data generated or analysed during this study are included in the published article and Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2039052 (3a), 2039053 (4a), 2039054 (3ac), 2039055 (4ac) and 2039056 (4ac′). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are available from the authors upon reasonable request.
References
Diels, O. & Alder, K. Syntheses in the hydroaromatic series [in German]. Justus Liebigs Ann. Chem. 460, 98–122 (1928).
Hoffmann, R. & Woodward, R. B. The conservation of orbital symmetry. Acc. Chem. Res. 1, 17–22 (1968).
Corey, E. J. Catalytic enantioselective Diels–Alder reactions: methods, mechanistic fundamentals, pathways and applications. Angew. Chem. Int. Ed. 41, 1650–1667 (2002).
Nicolaou, K. C., Snyder, S. A., Montagnon, T. M. & Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1698 (2002).
Ose, T. et al. Insight into a natural Diels–Alder reaction from the structure of macrophomate synthase. Nature 422, 185–189 (2003).
Chou, T. & Hung, S.-C. Selective cross Diels–Alder reaction of 2-(phenylsulfonyl) 1,3-dienes. J. Org. Chem. 53, 3020–3027 (1988).
Barco, A. et al. Generation and cycloaddition reactions of 3-substituted-2-nitro-1,3-dienes. Tetrahedron 52, 9275–9288 (1996).
Teyssot, M.-L., Lormier, A.-T., Chataigner, I. & Piettre, S. R. Cross-Diels–Alder reaction of 6-oxo-1-sulfonyl-1,6-dihydropyridine-3-carboxylates. J. Org. Chem. 72, 2364–2373 (2007).
Chou, S.-S. P. & Chen, P.-W. Cycloaddition reaction of 4-sulfur-substituted dihydro-2-pyridones and 2-pyridones with conjugated dienes. Tetrahedron 64, 1879–1887 (2008).
Pham, H. V. & Houk, K. N. Diels–Alder reactions of allene with benzene and butadiene: concerted, stepwise, and ambimodal transition states. J. Org. Chem. 79, 8968–8976 (2014).
Caramella, P., Quadreil, P. & Toma, L. An unexpected bispericyclic transition structure leading to 4+2 and 2+4 cycloadducts in the endo dimerization of cyclopentadiene. J. Am. Chem. Soc. 124, 1130–1131 (2002).
Hare, S. R. & Tantillo, D. J. Post-transition state bifurcations gain momentum – current state of the field. Pure Appl. Chem. 6, 679–698 (2017).
Ussing, B. R., Hang, C. & Singleton, D. A. Dynamic effects on the periselectivity, rate, isotope effects, and mechanism of cycloadditions of ketenes with cyclopentadiene. J. Am. Chem. Soc. 128, 7594–7607 (2006).
Thomas, J. B., Waas, J. R., Harmata, M. & Singleton, D. A. Control elements in dynamically determined selectivity on a bifurcating surface. J. Am. Chem. Soc. 130, 14544–14555 (2008).
Hong, Y. J. & Tantillo, D. J. A potential energy surface bifurcation in terpene biosynthesis. Nat. Chem. 1, 384–389 (2009).
Katori, T., Itoh, S., Sato, M. & Yamataka, H. Reaction pathways and possible path bifurcation for the Schmidt reaction. J. Am. Chem. Soc. 132, 3413–3422 (2010).
Garayalde, D., Gómez-Bengoa, E., Huang, X., Goeke, A. & Nevado, C. Mechanistic insights in gold-stabilized nonclassical carbocations: gold-catalyzed rearrangemement of 3-cyclopropyl propargylic acetates. J. Am. Chem. Soc. 132, 4720–4730 (2010).
Wang, Z. J., Benitez, D., Tkatchouk, E., Goddard, W. A. III & Toste, F. D. Mechanistic study of gold(I)-catalyzed intermolecular hydroamination of allenes. J. Am. Chem. Soc. 132, 13064–13071 (2010).
Hansen, J. H. et al. On the mechanism and selectivity of the combined C−H activation/Cope rearrangement. J. Am. Chem. Soc. 133, 5076–5085 (2011).
Nieves-Quinones, Y. & Singleton, D. A. Dynamics and the regiochemistry of nitration of toluene. J. Am. Chem. Soc. 138, 15167–15176 (2016).
Çelebi-Ölçüm, N., Ess, D. H., Aviyente, V. & Houk, K. N. Lewis acid catalysis alters shapes and products of bis-pericyclic Diels–Alder transition states. J. Am. Chem. Soc. 129, 4528–4529 (2007).
Yu, P. et al. Mechanisms and origins of periselectivity of the ambimodal [6 + 4] cycloadditions of tropone to dimethylfulvene. J. Am. Chem. Soc. 139, 8251–8258 (2017).
Chen, S., Yu, P. & Houk, K. N. Ambimodal dipolar/Diels–Alder cycloaddition transition states involving proton transfers. J. Am. Chem. Soc. 140, 18124–18131 (2019).
Xue, X.-S. et al. Ambimodal trispericyclic transition state and dynamic control of periselectivity. J. Am. Chem. Soc. 141, 1217–1221 (2019).
Liu, F., Chen, Y. & Houk, K. N. Huisgen’s 1,3-dipolar cycloadditions to fulvenes proceed via ambimodal [6+4]/[4+2] transition states. Angew. Chem. Int. Ed. 59, 12412–12416 (2020).
Kim, K., Ruszczycky, M. W., Choi, S.-H., Liu, Y.-N. & Liu, H.-W. Enzyme-catalyzed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 473, 109–112 (2011).
Partel, A. et al. Dynamically complex [6+4] and [4+2] cycloadditions in the biosynthesis of spinosyn A. J. Am. Chem. Soc. 138, 3631–3634 (2016).
Ohashi, M. et al. SAM-dependent enzyme-catalyzed pericyclic reactions in natural product biosynthesis. Nature 549, 502–506 (2016).
Zhang, B. et al. Enzyme-catalysed [6+4] cycloadditions in the biosynthesis of natural products. Nature 568, 122–126 (2019).
Zhang, Z. et al. Enzyme-catalyzed inverse-electron demand Diels–Alder reaction in the biosynthesis of antifungal ilicicolin H. J. Am. Chem. Soc. 141, 5659–5663 (2019).
Si, X.-G., Zhang, Z.-M., Zheng, C.-G., Li, Z.-T. & Cai, Q. Enantioselective synthesis of cis-decalin derivatives by the inverse-electron-demand Diels–Alder reaction of 2-pyrones. Angew. Chem. Int. Ed. 59, 18412–18417 (2020).
Gregson, R. P. & Mirrington, R. N. Stereospecific total synthesis of (±)-α-amorphene. J. Chem. Soc. Chem. Commun. 598–599 (1973).
Markó, I. E. & Evans, G. R. Catalytic, enantioselective, inverse electron-demand Diels–Alder (IEDDA) reactions of 3-carbomethoxy-2-pyrone (3-CMP). Tetrahedron Lett. 35, 2771–2774 (1994).
Posner, G. H., Eydoux, F., Lee, J. K., Bull, D. S. & Dai, H. Binaphthol-titanium-promoted, highly enantiocontrolled, Diels−Alder cycloadditions of electronically matched 2-pyrones and vinyl ethers: streamlined asymmetric synthesis of an A-ring precursor to physiologically active 1α-hydroxyvitamin D3 steroids. Tetrahedron Lett. 35, 7541–7544 (1994).
Markó, I. E., Warriner, S. L. & Augustynes, B. Radical-initiated, skeletal rearrangements of bicyclo[2.2.2]lactones. Org. Lett. 2, 3123–3125 (2000).
Burch, P. et al. Total synthesis of gelsemiol. Chem. Eur. J. 19, 2589–2591 (2013).
Zhou, Y., Zhou, Z., Du, W. & Chen, Y. Asymmetric inverse-electron-demand Diels–Alder reaction of 2-pyrone and 2,5-dienones via HOMO-activation. Acta Chim. Sin. 76, 382–386 (2018).
Liang, X.-W. et al. Enantioselective synthesis of arene cis-dihydrodiols from 2-pyrones. Angew. Chem. Int. Ed. 58, 14562–14567 (2019).
Cole, C., Fuentes, L. & Snyder, S. A. Asymmetric pyrone Diels–Alder reactions enabled by dienamine catalysis. Chem. Sci. 11, 2175–2180 (2020).
Liao, S., Sun, X.-L. & Tang, Y. Side arm strategy for catalyst design: modifying bisoxazolines for remote control of enantioselection and related. Acc. Chem. Res. 47, 2260–2272 (2014).
Evans, D. A., Johnson, J. S. & Olhava, E. J. Enantioselective synthesis of dihydropyrans. Catalysis of hetero Diels–Alder reactions by bis(oxazoline) copper(II) complexes. J. Am. Chem. Soc. 122, 1635–1649 (2000).
Yang, Z. et al. Relationships between product ratios in ambimodal pericyclic reactions and bond lengths in transition structures. J. Am. Chem. Soc. 140, 3061–3067 (2018).
Corey, E. J. & Loh, T.-P. First application of attractive intramolecular interactions to the design of chiral catalysts for highly enantioselective Diels–Alder reactions. J. Am. Chem. Soc. 113, 8966–8967 (1991).
Houk, K. N. & Luckus, L. J. Cycloadditions of dienes to fulvenes. J. Org. Chem. 38, 3836–3843 (1973).
Turconi, J. et al. Semisynthetic artemisinin, the chemical path to industrial production. Org. Process Res. Dev. 18, 417–422 (2014).
Barluenga, J., Fernández-Simón, J. L., Concellón, J. M. & Yu, M. Facile one-pot transformation of carboxylic acid chlorides into 2-substituted allyl alcohols or epichlorohydrins. Chem. Soc. Perkin Trans. 1 77–80 (1989).
Littleson, M. M. et al. Synthetic approaches to coronafacic acid, coronamic acid, and coronatine. Synthesis 48, 3429–3448 (2016).
Nara, S., Toshima, H. & Ichihara, A. Asymmetric total synthesis of (+)-coronafacic acid and (+)-coronatine, phytotoxins isolated from Pseudomonas syringae pathovars. Tetrahedron 53, 9509–9524 (1997).
Arai, T., Sasai, H., Yamaguchi, K. & Shibasaki, M. Regioselective catalytic asymmetric reaction of Horner-Wadsworth-Emmons reagents with enones: the odyssey of chiral aluminum catalysts. J. Am. Chem. Soc. 120, 441–442 (1998).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (grant nos. 21801043 and 22071030 to Q.C.), the “1000-Youth Talents Plan” (to Q.C.), Fudan University (start-up grant to Q.C.), the National Science Foundation of the USA (CHE-1764328 to K.N.H) and the Natural Science Foundation of Zhejiang Province (grant no. LY20B020010 to L.Y.) for financial support. L.Y. is grateful for additional funding from the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
M.-M.X. developed the ambimodal cross-Diels−Alder reaction, optimized the reaction conditions, conducted the control experiments, evaluated the scope of the reaction and applied this reaction to the synthesis of the natural products artemisinic acid and coronafacic acid; L.Y. and X.C. performed the DFT calculations; K.T. helped to evaluate the scope of the reaction; Q.-T.L. helped in the synthesis of coronafacic acid; K.N.H. supervised the computational studies; Q.C. conceived and directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Luis Domingo, Aurélien de la Torre and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Mechanistic Studies, Tables 1–4, Figs. 1 and 2, references and spectra.
Supplementary Data 1
Crystallographic data of compound 3a (CCDC 2039052).
Supplementary Data 2
Crystallographic data of compound 4a (CCDC 2039053).
Supplementary Data 3
Crystallographic data of compound 3ac (CCDC 2039054).
Supplementary Data 4
Crystallographic data of compound 4ac (CCDC 2039055).
Supplementary Data 5
Crystallographic data of compound 4ac′ (CCDC 2039056).
Rights and permissions
About this article
Cite this article
Xu, MM., Yang, L., Tan, K. et al. An enantioselective ambimodal cross-Diels–Alder reaction and applications in synthesis. Nat Catal 4, 892–900 (2021). https://doi.org/10.1038/s41929-021-00687-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00687-x