Abstract
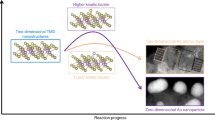
Transition metal dichalcogenide (TMD) nanomaterials, especially the mono- or few-layer ones, have received extensive research interest owing to their versatile properties, ranging from true metals (e.g., NbS2 and VSe2) and semimetals (e.g., WTe2 and TiSe2) to semiconductors (e.g., MoS2 and We2) and insulators (e.g., HfS2). Therefore, the reliable production of these nanomaterials with atomically thin thickness and laterally uniform dimension is essential for their promising applications in transistors, photodetectors, electroluminescent devices, catalysis, energy conversion, environment remediation, biosensing, bioimaging, and so on. Recently, the electrochemical lithium ion intercalation-based exfoliation method has emerged as a mature, efficient and promising strategy for the high-yield production of mono- or few-layer TMD nanosheets; monolayer MoS2 (yield of 92%), monolayer TaS2 (yield of 93%) and bilayer TiS2 (yield of 93%) with lateral dimensions of ~1 µm (refs. 1,2,3). This Protocol describes the details of experimental procedures for the high-yield synthesis of mono- or few-layer TMDs and other inorganic nanosheets such as MoS2, WS2, TiS2, TaS2, ZrS2, graphene, h-BN, NbSe2, WSe2, Sb2Se3 and Bi2Te3 by using the electrochemical lithium ion intercalation-based exfoliation method, which involves the electrochemical intercalation of lithium ions into layered inorganic crystals and a mild sonication process. The whole protocol takes 26–38 h for the successful production of ultrathin inorganic nanosheets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Zeng, Z. et al. Single-layer semiconducting nanosheets: high-yield preparation and device fabrication. Angew. Chem. Int. Ed. 50, 11093–11097 (2011).
Zeng, Z. et al. An effective method for the fabrication of few-layer-thick inorganic nanosheets. Angew. Chem. Int. Ed. 51, 9052–9056 (2012).
Zeng, Z., Tan, C., Huang, X., Bao, S. & Zhang, H. Growth of noble metal nanoparticles on single-layer TiS2 and TaS2 nanosheets for hydrogen evolution reaction. Energy Environ. Sci. 7, 797–803 (2014).
Joensen, P., Frindt, R. F. & Morrison, S. R. Single-layer MoS2. Mater. Res. Bull. 21, 457–461 (1986).
Desai, S. B. et al. MoS2 transistors with 1-nanometer gate lengths. Science 354, 99–102 (2016).
Kong, L. et al. Doping-free complementary WSe2 circuit via van der Waals metal integration. Nat. Commun. 11, 1866 (2020).
Li, J. et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nature 579, 368–374 (2020).
Zhao, B. et al. High-order superlattices by rolling up van der Waals heterostructures. Nature 591, 385–390 (2021).
Yu, Y. et al. Gate-tunable phase transitions in thin flakes of 1T-TaS2. Nat. Nanotechnol. 10, 270–276 (2015).
Mak, K. F. & Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 10, 216–226 (2016).
Velusamy, D. B. et al. Flexible transition metal dichalcogenide nanosheets for band-selective photodetection. Nat. Commun. 6, 8063 (2015).
Wang, H., Zhang, C., Chan, W., Tiwari, S. & Rana, F. Ultrafast response of monolayer molybdenum disulfide photodetectors. Nat. Commun. 6, 8831 (2015).
Binder, J. et al. Upconverted electroluminescence via Auger scattering of interlayer excitons in van der Waals heterostructures. Nat. Commun. 10, 2335 (2019).
Lorchat, E. et al. Filtering the photoluminescence spectra of atomically thin semiconductors with graphene. Nat. Nanotechnol. 15, 283–288 (2020).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013).
Liu, Y. et al. Self-optimizing, highly surface-active layered metal dichalcogenide catalysts for hydrogen evolution. Nat. Energy 2, 17127 (2017).
Voiry, D., Shin, H. S., Loh, K. P. & Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2, 0105 (2018).
Yu, Y. et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 10, 638–643 (2018).
Lin, L. et al. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 19, 408–423 (2019).
Liu, C. et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 11, 1098–1104 (2016).
Zhu, C. et al. Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. J. Am. Chem. Soc. 135, 5998–6001 (2013).
Kalantar-zadeh, K. et al. Two-dimensional transition metal dichalcogenides in biosystems. Adv. Funct. Mater. 25, 5086–5099 (2015).
Manzeli, S., Ovchinnikov, D., Pasquier, D., Yazyev, O. V. & Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2, 17033 (2017).
Xu, X., Yao, W., Xiao, D. & Heinz, T. F. Spin and pseudospins in layered transition metal dichalcogenides. Nat. Phys. 10, 343–350 (2014).
Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Chhowalla, M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013).
Xi, X. et al. Ising pairing in superconducting NbSe2 atomic layers. Nat. Phys. 12, 139–143 (2016).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011).
Mak, K. F., Lee, C., Hone, J., Shan, J. & Heinz, T. F. Atomically thin MoS2: a new direct-gap semiconductor. Phys. Rev. Lett. 105, 136805 (2010).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2. Nano Lett. 10, 1271–1275 (2010).
Lin, Z. et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 562, 254–258 (2018).
Cullen, P. L. et al. Ionic solutions of two-dimensional materials. Nat. Chem. 9, 244–249 (2017).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Kappera, R. et al. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 13, 1128–1134 (2014).
Li, J. et al. Printable two-dimensional superconducting monolayers. Nat. Mater. 20, 181–187 (2021).
Zheng, J. et al. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat. Commun. 5, 2995 (2014).
Chou, S. S. et al. Controlling the metal to semiconductor transition of MoS2 and WS2 in solution. J. Am. Chem. Soc. 137, 1742–1745 (2015).
Chou, S. S. et al. Chemically exfoliated MoS2 as near-infrared photothermal agents. Angew. Chem. Int. Ed. 52, 4160–4164 (2013).
Chou, S. S. et al. Ligand conjugation of chemically exfoliated MoS2. J. Am. Chem. Soc. 135, 4584–4587 (2013).
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011).
Feng, J. et al. Metallic few-layered VS2 ultrathin nanosheets: high two-dimensional conductivity for in-plane supercapacitors. J. Am. Chem. Soc. 133, 17832–17838 (2011).
Wan, C. et al. Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2. Nat. Mater. 14, 622–627 (2015).
Zhou, J. et al. A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018).
Wang, H. et al. High-quality monolayer superconductor NbSe2 grown by chemical vapour deposition. Nat. Commun. 8, 394 (2017).
Lin, H. et al. Growth of environmentally stable transition metal selenide films. Nat. Mater. 18, 602–607 (2019).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 12, 754–759 (2013).
Lee, Y.-H. et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 24, 2320–2325 (2012).
Feldman, Y., Wasserman, E., Srolovitz, D. J. & Tenne, R. High-rate, gas-phase growth of MoS2 nested inorganic fullerenes and nanotubes. Science 267, 222–225 (1995).
van der Zande, A. M. et al. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 12, 554–561 (2013).
Shi, J. et al. Two-dimensional metallic tantalum disulfide as a hydrogen evolution catalyst. Nat. Commun. 8, 958 (2017).
Gong, Y. et al. Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics. Nat. Nanotechnol. 13, 294–299 (2018).
Jung, W. et al. Colloidal synthesis of single-layer MSe2 (M = Mo, W) nanosheets via anisotropic solution-phase growth approach. J. Am. Chem. Soc. 137, 7266–7269 (2015).
Yoo, D., Kim, M., Jeong, S., Han, J. & Cheon, J. Chemical synthetic strategy for single-layer transition-metal chalcogenides. J. Am. Chem. Soc. 136, 14670–14673 (2014).
Jeong, S., Yoo, D., Jang, J.-T., Kim, M. & Cheon, J. Well-defined colloidal 2-D layered transition-metal chalcogenide nanocrystals via generalized synthetic protocols. J. Am. Chem. Soc. 134, 18233–18236 (2012).
Mahler, B., Hoepfner, V., Liao, K. & Ozin, G. A. Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 136, 14121–14127 (2014).
Xie, J. et al. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 135, 17881–17888 (2013).
Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 9, 9451–9469 (2015).
Sun, L. et al. Chemical vapour deposition. Nat. Rev. Methods Prim. 1, 5 (2021).
Lai, Z. et al. Metastable 1T′-phase group VIB transition metal dichalcogenide crystals. Nat. Mater. https://doi.org/10.1038/s41563-021-00971-y (2021).
Tan, C. et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117, 6225–6331 (2017).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Tan, C. et al. Liquid-phase epitaxial growth of two-dimensional semiconductor hetero-nanostructures. Angew. Chem. Int. Ed. 54, 1841–1845 (2015).
Lin, Z. et al. High-yield exfoliation of 2D semiconductor monolayers and reassembly of organic/inorganic artificial superlattices. Chem 7, 1887–1902 (2021).
Kumbhakar, P. et al. Emerging 2D metal oxides and their applications. Mater. Today 45, 142–168 (2021).
Zhu, J. et al. Layer-by-layer assembled 2D montmorillonite dielectrics for solution-processed electronics. Adv. Mater. 28, 63–68 (2016).
Yang, R. et al. MnO2-based materials for environmental applications. Adv. Mater. 33, 2004862 (2021).
Zhang, Q., Mei, L., Cao, X., Tang, Y. & Zeng, Z. Intercalation and exfoliation chemistries of transition metal dichalcogenides. J. Mater. Chem. A 8, 15417–15444 (2020).
He, Q. et al. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 8, 2994–2999 (2012).
Yin, Z. et al. Memory devices using a mixture of MoS2 and graphene oxide as the active layer. Small 9, 727–731 (2013).
Huang, X. et al. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 4, 1444 (2013).
Mei, L. et al. Size-selective synthesis of platinum nanoparticles on transition-metal dichalcogenides for the hydrogen evolution reaction. Chem. Commun. https://doi.org/10.1039/D0CC08091H (2021).
Yin, Z. et al. Preparation of MoS2–MoO3 hybrid nanomaterials for light-emitting diodes. Angew. Chem. Int. Ed. 53, 12560–12565 (2014).
Zeng, Z. et al. In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy. Nano Lett. 15, 5214–5220 (2015).
Yang, H., Kim, S. W., Chhowalla, M. & Lee, Y. H. Structural and quantum-state phase transitions in van der Waals layered materials. Nat. Phys. 13, 931–937 (2017).
Voiry, D., Mohite, A. & Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 44, 2702–2712 (2015).
Li, W., Qian, X. & Li, J. Phase transitions in 2D materials. Nat. Rev. Mater. https://doi.org/10.1038/s41578-021-00304-0 (2021).
Heising, J. & Kanatzidis, M. G. Structure of restacked MoS2 and WS2 elucidated by electron crystallography. J. Am. Chem. Soc. 121, 638–643 (1999).
Voiry, D., Drummond, C. & Pénicaud, A. Portrait of carbon nanotube salts as soluble polyelectrolytes. Soft Matter 7, 7998–8001 (2011).
Acknowledgements
Z.Z. thanks the Start-Up Grant from CityU (CityU9610435) and ECS scheme (CityU9048163) from RGC in Hong Kong, and the Basic Research Project from Shenzhen Science and Technology Innovation Committee in Shenzhen, China (JCYJ20210324134012034). H.S.S. acknowledges support by research funds (2020M3D1A1110548 and 2019M1A2A2065616) through NRF, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Z.Z. developed the protocol and performed the experiments. R.Y., L.M. and Z.Z. drafted the manuscript. R.Y., L.M. and Q.Z. performed some experiments. H.S.S. and D.V. provided constructive comments on the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Huang, X. et al. Nat. Commun. 4, 1444 (2013): https://www.nature.com/articles/ncomms2472#citeas
Zhu, C. et al. J. Am. Chem. Soc. 135, 5998–6001 (2013): https://pubs.acs.org/doi/10.1021/ja4019572
Mei, L. et al. Chem. Commun. 57, 2879 (2021): https://doi.org/10.1039/D0CC08091H
Key data used in this protocol
Zeng, Z. et al. Angew. Chem. Int. Ed. 50, 11093–11097 (2011): https://onlinelibrary.wiley.com/doi/10.1002/anie.201106004
Zeng, Z. et al. Angew. Chem. Int. Ed. 51, 9052–9056 (2012): https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201204208
Zeng, Z. et al. Energy Environ. Sci. 7, 797–803 (2014): https://doi.org/10.1039/C3EE42620C
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Supplementary Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Yang, R., Mei, L., Zhang, Q. et al. High-yield production of mono- or few-layer transition metal dichalcogenide nanosheets by an electrochemical lithium ion intercalation-based exfoliation method. Nat Protoc 17, 358–377 (2022). https://doi.org/10.1038/s41596-021-00643-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00643-w
This article is cited by
-
Metal telluride nanosheets by scalable solid lithiation and exfoliation
Nature (2024)
-
Photocatalytic applications and modification methods of two-dimensional nanomaterials: a review
Tungsten (2024)
-
Recent advances on liquid intercalation and exfoliation of transition metal dichalcogenides: From fundamentals to applications
Nano Research (2024)
-
Photoelectric and Magnetic Variation of Transition Metal-Doped Monolayer TiS2: A First-Principles Calculation
Journal of Superconductivity and Novel Magnetism (2024)
-
Synthesis of atomically thin sheets by the intercalation-based exfoliation of layered materials
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.